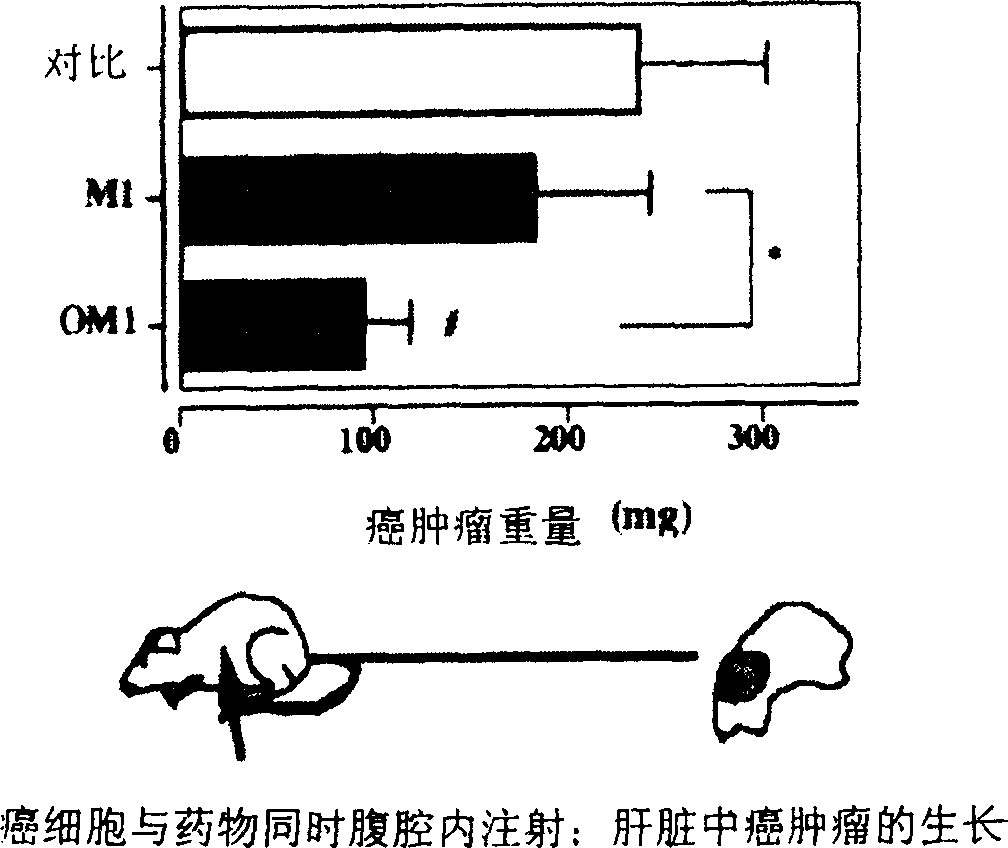
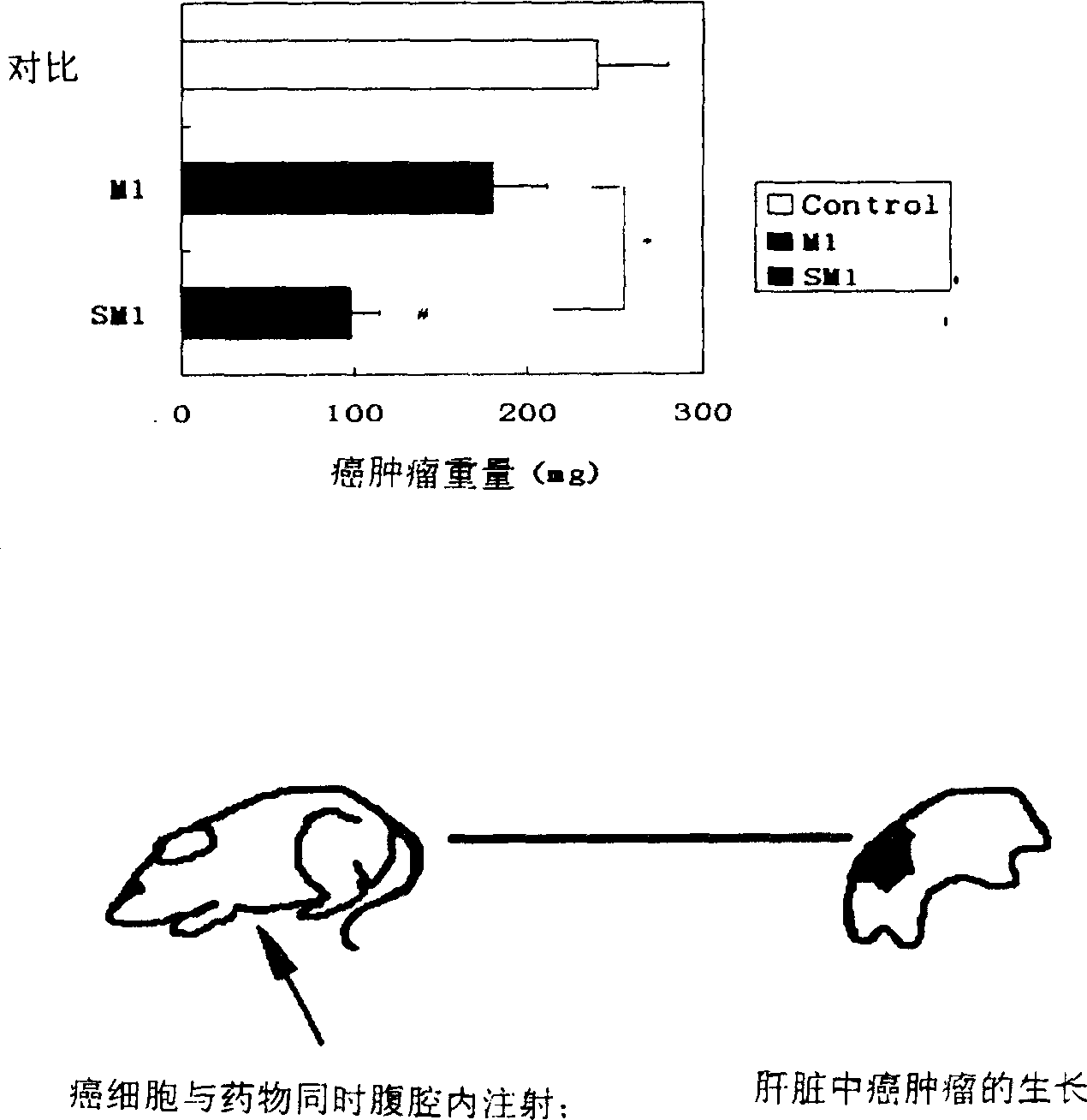
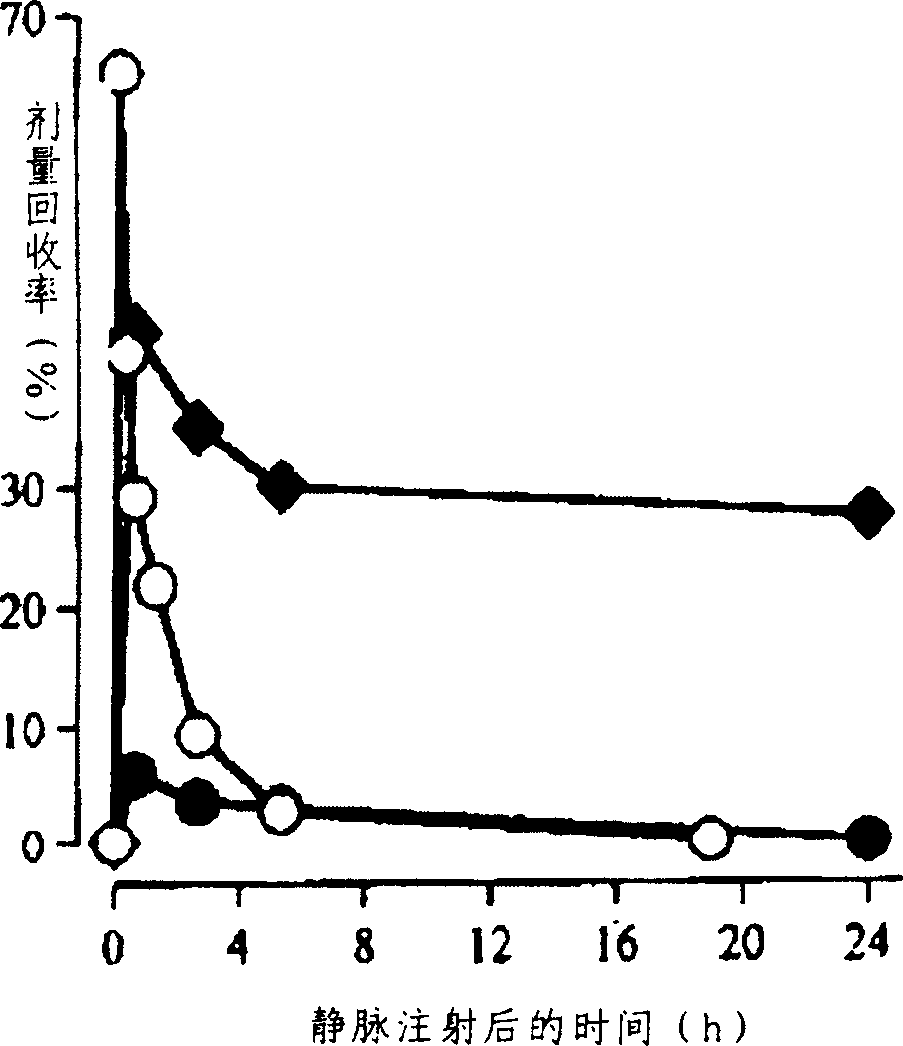
Fatty acid ester compounds of ginsenoside secondary glycoside compound K and method for preparing same
A technology of stearate and protopanaxadiol, which is applied in the fields of medical preparations containing active ingredients, sugar derivatives, organic chemistry, etc., and can solve problems such as no artificial synthetic products reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Take M130g and dissolve in 1000ml ethyl acetate, add 1000ml water-saturated sodium bicarbonate under stirring condition, add oleic acid chloride or stearic acid chloride 720g under ice-water bath condition, stir overnight at room temperature. Then use a separatory funnel to separate the ethyl acetate layer from the water layer, and repeatedly extract the water layer with ethyl acetate, combine the ethyl acetate, centrifuge (3000rpm), and wash the supernatant with water repeatedly, and the ethyl acetate is at low temperature (20 -30°C) for recovery under reduced pressure, the residue was dissolved in methanol, filtered, and the methanol solution was concentrated under reduced pressure to obtain dry matter.
Embodiment 2
[0126] Take M140g and dissolve in 1200ml ethyl acetate, add 1000ml water-saturated sodium bicarbonate under stirring condition, add oleic acid chloride or stearic acid chloride 700g under ice-water bath condition, stir overnight at room temperature. Then use a separatory funnel to separate the ethyl acetate layer from the water layer, and repeatedly extract the water layer with ethyl acetate, combine the ethyl acetate, centrifuge (3000rpm), wash the supernatant with water repeatedly, and store the ethyl acetate at a low temperature of 20°C ) under reduced pressure recovery, the residue was dissolved in methanol, filtered, and the methanol solution was concentrated under reduced pressure to obtain dry matter.
Embodiment 3
[0128] Take M150g and dissolve in 1500ml ethyl acetate, add 1500ml water-saturated sodium bicarbonate under stirring condition, add oleic acid chloride or stearic acid chloride 720g under ice-water bath condition, stir overnight at room temperature. Then use a separatory funnel to separate the ethyl acetate layer from the water layer, and repeatedly extract the water layer with ethyl acetate, combine the ethyl acetate, centrifuge (3000rpm), and wash the supernatant with water repeatedly. Recover under reduced pressure, dissolve the residue with methanol, filter, and concentrate the methanol solution under reduced pressure to obtain dry matter.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com