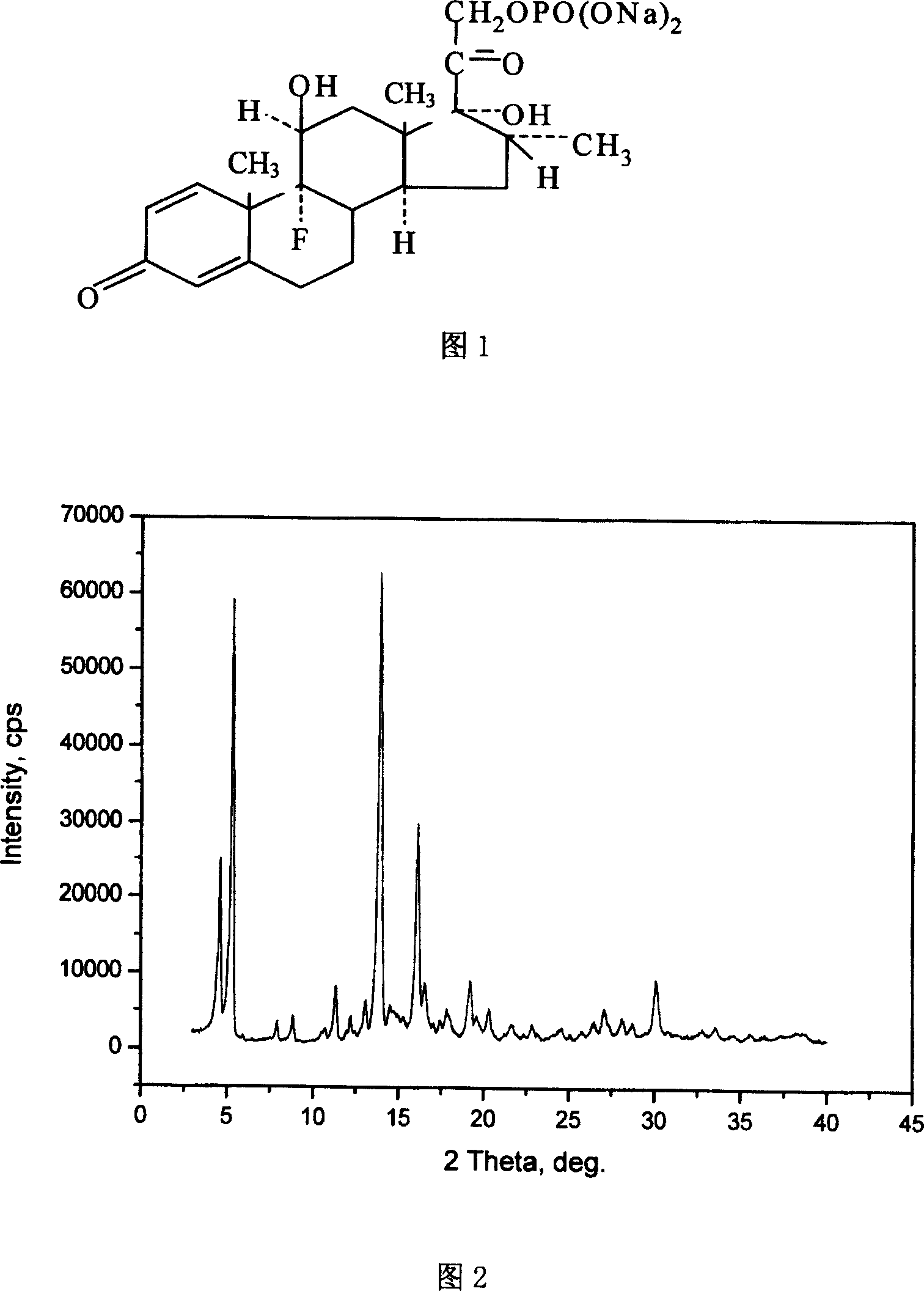
Dexamethasone sodium phosphate crystal form and its crystallization preparation method
A technology of dexamethasone sodium phosphate and crystal form, which is applied to the crystal form of dexamethasone sodium phosphate and the field of crystal preparation, can solve the problems affecting product purity, storage time, different heat resistance and the like, achieves high crystallinity, Easy to filter, large particle size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 120g of dexamethasone phosphate into a three-necked flask, add 2000ml of ethanol and 6g of activated carbon at the same time, stir and dissolve at a constant temperature of 40°C, and filter after continuous stirring for 60 minutes. The filtrate is moved in the crystallizer, and configuration concentration is the sodium hydroxide solution of 100g / L simultaneously. Then sodium hydroxide solution is added into the crystallizer for reaction, and the pH value at the end of the reaction process is controlled at 11.0. Add about 4000 ml of diethyl ether-water mixed solvent to the reacted dexamethasone sodium phosphate solution while stirring at 20° C. to crystallize. After the crystallization is finished, carry out centrifugation, wash with acetone, and dry the obtained crystal product at 70° C. and a pressure of 0.1 MPa for 6 hours, and then dry at this temperature for 15 hours at a pressure of 0.008 MPa. The content of the final crystal product is 99.3%, the quality conf...
Embodiment 2
[0030] Add 12g of dexamethasone phosphate into a three-necked flask, add 120ml of ethanol and 1.2g of activated carbon at the same time, stir and dissolve at a constant temperature of 40°C, and filter after continuous stirring for 30 minutes. The filtrate is moved in the crystallizer, and configuration concentration is the sodium hydroxide solution of 100g / L simultaneously. Then sodium hydroxide solution is added into the crystallizer for reaction, and the pH value at the end of the reaction process is controlled at 10.5. Add about 360 ml of diethyl ether-water mixed solvent to the reacted dexamethasone sodium phosphate solution while stirring at 25° C. for crystallization. Centrifuge after crystallization, wash with ethanol, dry the obtained crystal product at 50° C. and a pressure of 0.1 MPa for 6 hours, and then dry at this temperature for 14 hours at 0.002 MPa. The content of the final crystal product is 99.3%, and the quality conforms to the latest pharmacopoeia standard...
Embodiment 3
[0032] Add 6g of dexamethasone phosphate into a three-necked flask, add 50ml of methanol and 0.5g of activated carbon at the same time, stir and dissolve at a constant temperature of 45°C, and filter after continuous stirring for 60 minutes. The filtrate is moved into the crystallizer, and the sodium hydroxide solution of 250g / L is configured simultaneously. Then sodium hydroxide solution is added into the crystallizer for reaction, and the pH value at the end of the reaction process is controlled at 9.5. Add about 300 ml of ethanol-water mixed solvent to the reacted dexamethasone sodium phosphate solution while stirring at 20° C. to crystallize. After the crystallization is completed, carry out centrifugation, wash with ethanol, and dry the obtained crystal product at a temperature of 80° C. and a pressure of 0.1 MPa for 5 hours, and then at this temperature of 0.009 MPa for 10 hours. The content of the final crystal product is 99.4%, and the quality conforms to the latest p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com