Human blood high density lipoprotein and its preparation method and use
A high-density lipoprotein and manufacturing method technology, applied in the field of biochemistry, can solve problems such as preparing HDL, and achieve the effects of inhibiting the occurrence of AS, promoting the reversal or regression of AS, and reducing the plaque area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
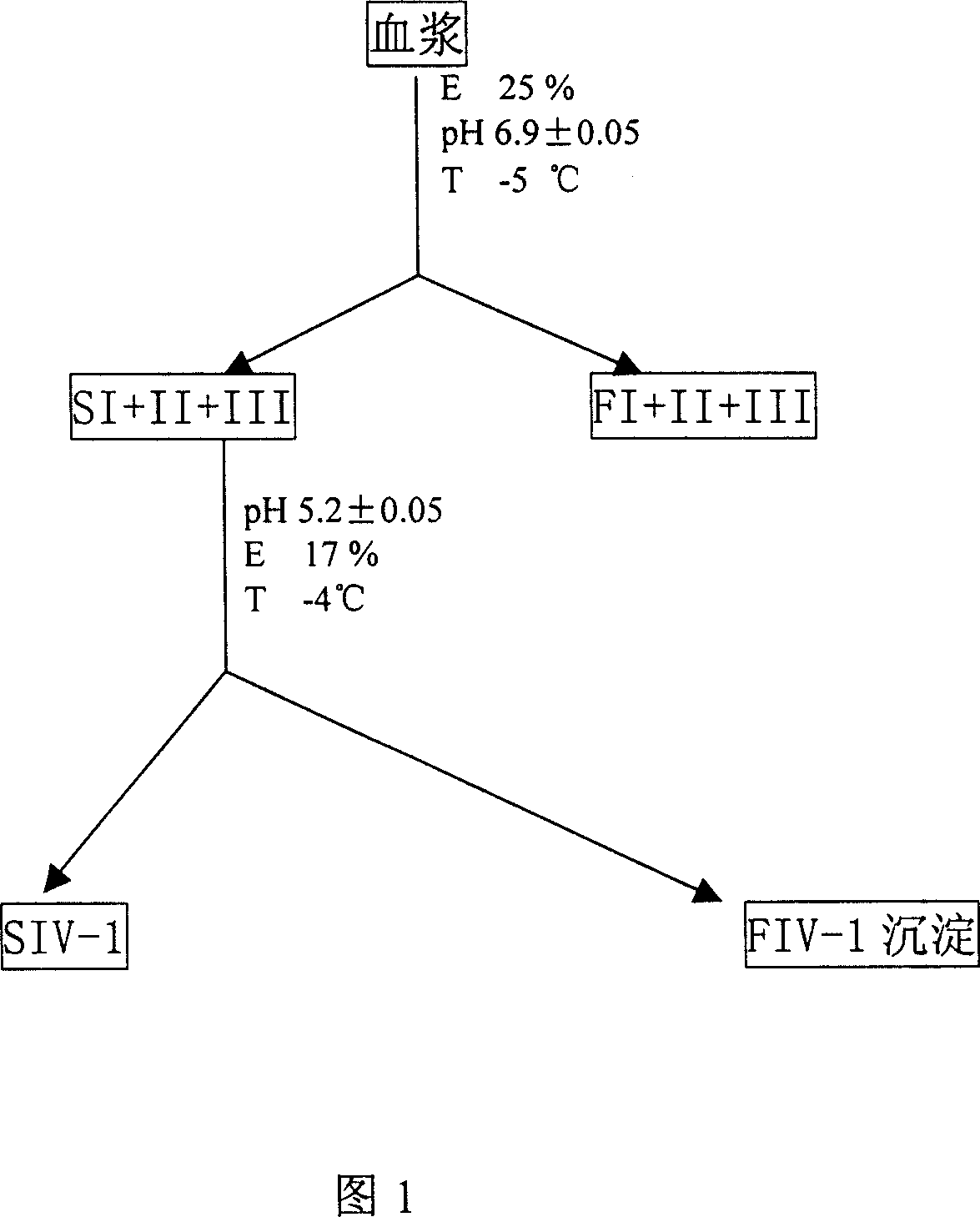
[0063] Take 1000L of raw plasma, add dropwise 25L of HAC / NaAC buffer solution with pH=4.0, adjust the pH of the mixture to=5.2, add 324L of 95% ethanol, stir at -5℃ for 2 hours, stand for 6 hours, use 142 type continuous Centrifuge at 14000r / min to get 1340L of supernatant and 50Kg of precipitation;
[0064] Add 630L of water for injection to the supernatant, drop 13.7L of the HAC / NaAC mixed solution with pH 4.0 to make the pH of the mixture 4.5, at this time the protein concentration is 1%, the ionic strength is 0.05, and stir for 2 hours at -3°C , Stand for 2 hours, use 142 continuous centrifuge, centrifuge at 14000r / min, get 9Kg of precipitate, discard the supernatant;
[0065] Add 19.8L of 30mmol / L sodium chloride solution to the precipitate, drop 14L of HAC / NaAC mixed solution with pH 4.0 to pH 5.5, stir at 0℃ for 4 hours, use 142 type continuous centrifuge, at 14000r / Centrifuge at min, discard the supernatant, get 4.65Kg of precipitate;
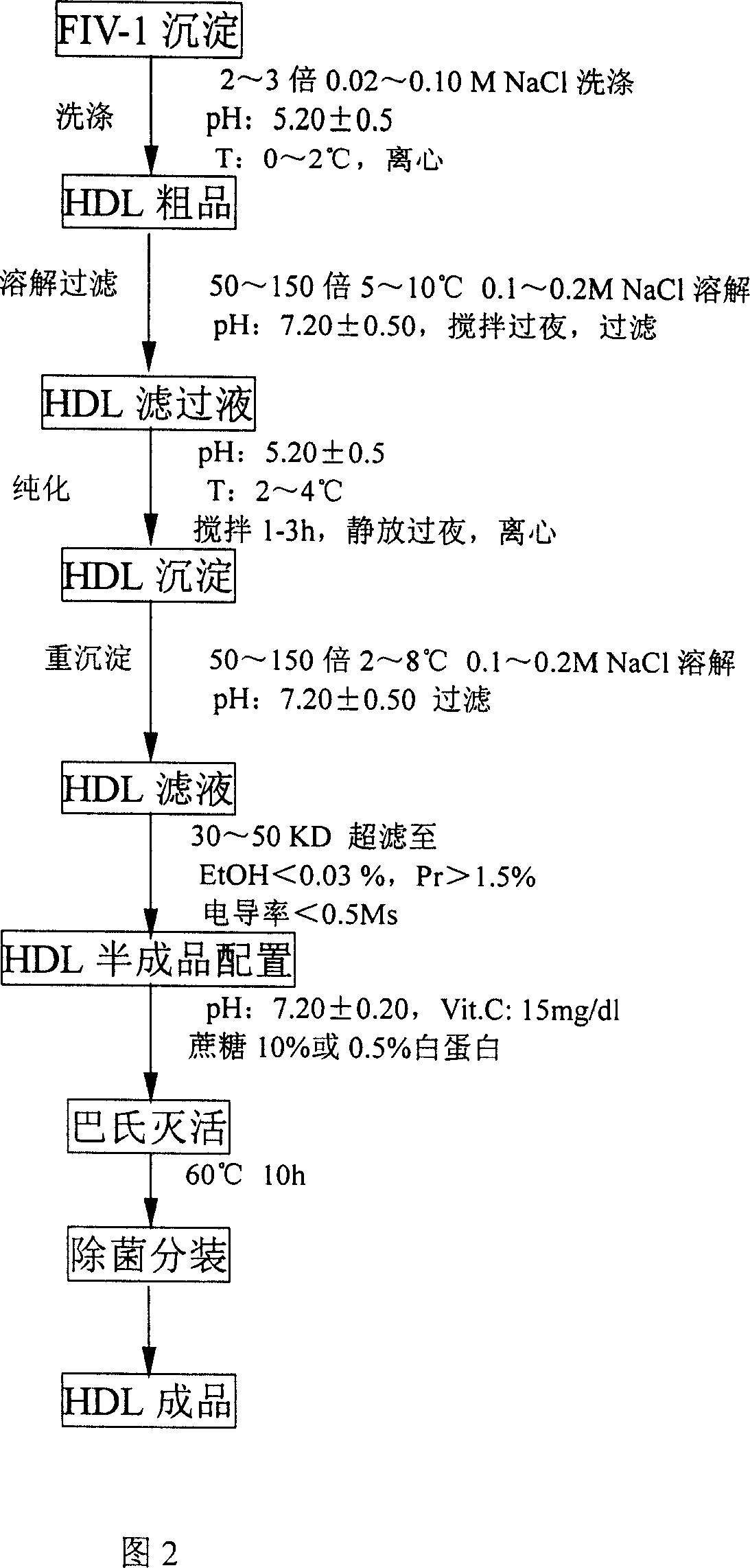
[0066] Add 160mmol / L sodium chlorid...
Embodiment 2
[0071] Take 1000L of raw plasma, add dropwise 24.5L of HAC / NaAC buffer solution with pH=4.0, adjust the pH of the mixed solution to 5.15, add 330L of 95% ethanol, stir for 2 hours at -4°C, stand for 8 hours, use type 142 Continuous centrifuge, centrifuge at 14000r / min, get 1270L of supernatant and 47Kg of precipitation;
[0072] Add 646L of water for injection to the supernatant, drop 14.3L of the HAC / NaAC mixed solution with pH 4.0 to make the pH of the mixture 5.2, the protein concentration is 1.68%, and the ionic strength is 0.07. Stir at -6°C for 2 hours , Let stand for 2 hours, use 142 continuous centrifuge, centrifuge at 14000r / min, get 9.5Kg of precipitate, discard the supernatant;
[0073] Add 19.0L of 30mmol / L sodium chloride solution to the precipitate, drop 13.7L of the HAC / NaAC mixed solution with pH 4.0 to make the pH 5.7, stir at 1℃ for 4 hours, use 142 type continuous centrifuge at 14000r Centrifuge at / min, discard the supernatant, and get 4.33Kg of precipitate;
...
Embodiment 3
[0079] Take 1000L of raw plasma, add dropwise 24.3L of HAC / NaAC buffer solution with pH=4.0, adjust the pH of the mixture to=5.22, add 304L of 95% ethanol, stir at -5°C for 2 hours, stand for 6 hours, use type 142 Continuous centrifuge, centrifuge at 14000r / min, get 1299L of supernatant and 45Kg of precipitation;
[0080] Supplement the supernatant with 611L of water for injection, dropwise add 14L of the HAC / NaAC mixed solution with pH 4.0 to make the pH of the mixture 5.1, the protein concentration is 1.68%, and the ionic strength is 0.07. Stir at -6°C for 2 hours. Let stand for 2 hours, use 142 continuous centrifuge, centrifuge at 14000r / min, get 8.9Kg of precipitate, discard the supernatant;
[0081] Add 18.9L of 30mmol / L sodium chloride solution to the precipitate, add 13.6L of HAC / NaAC mixed solution with pH 4.0 dropwise to make the pH 4.9, stir at 0℃ for 4 hours, use 142 type continuous centrifuge, at 14000r Centrifuge at / min, discard the supernatant, and get 4.05Kg of pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com