Mateirals and methods for alteration of enzyme and acetyl coA levels in plants
A technology of acetyl-CoA and plants, applied in the field of acetyl-CoA synthetase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
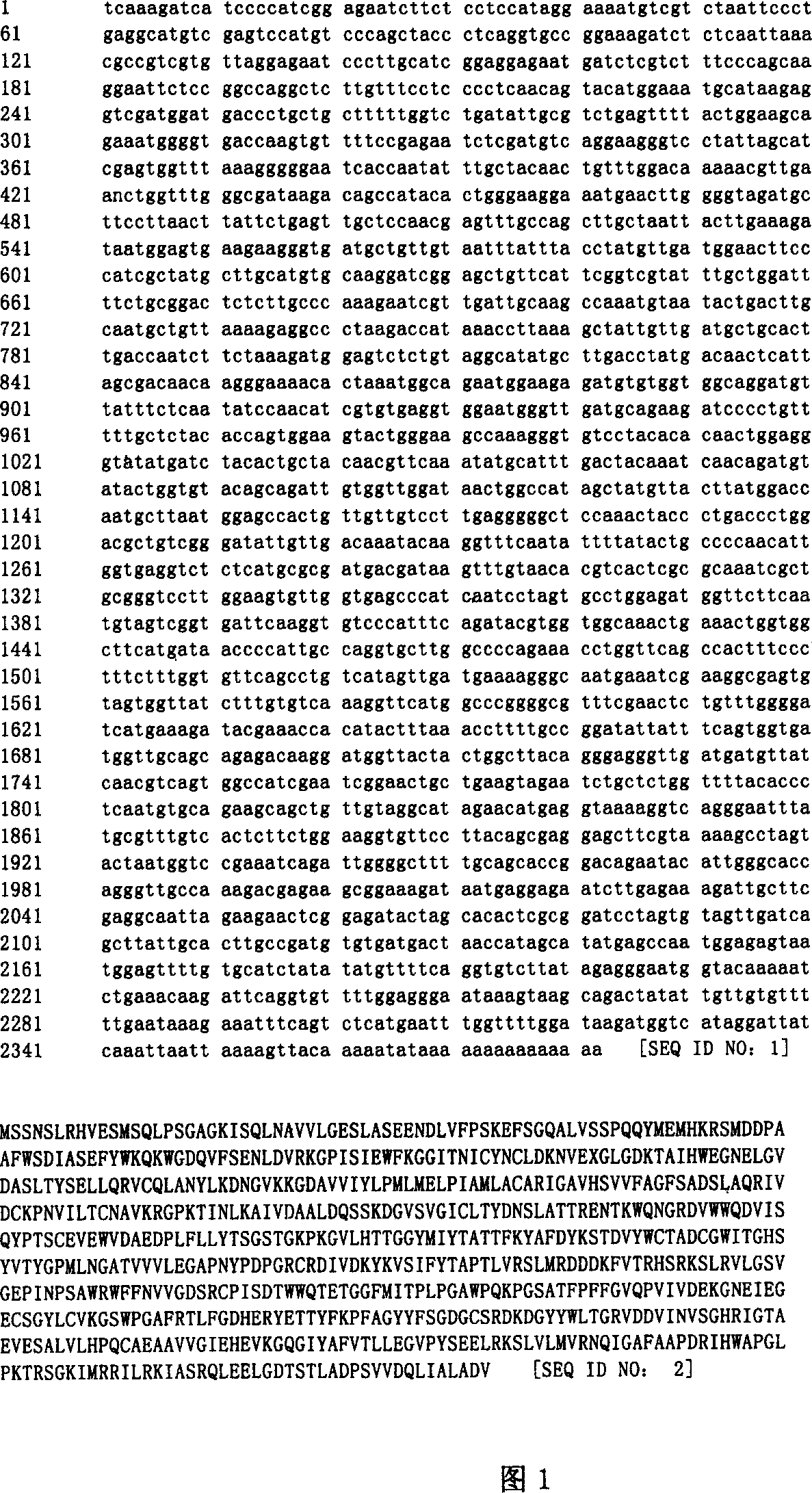
[0130] This example describes the cloning of ACS cDNA from Arabidopsis and comparison of its sequence with that of E. coli and S. cerevisiae.
[0131] The deduced amino acid sequences of the E. coli and S. cerevisiae ACS genes were searched using the program BLAST against the dbEST database (National Library of Medicine, National Institute of Health, Bethesda, Maryland). One Arabidopsisthaliana cDNA clone was identified as a possible ACS gene. This clone, 220J9T7 (accession number N38599), was obtained from the Arabidopsis Research Center for Biology (ARBC) at Ohio State University.
[0132] Plastid DNA of this clone was prepared and sequenced by the Biotechnology Instrumentation Facility of Iowa State University. Analysis of the sequence data found that this clone (J9) encoded approximately 40% of the ACSC-terminal portion. Repeated searches of dbEST failed to reveal any longer cloned sequences.
[0133] The remainder of the ACS gene was isolated using cRACE (Maruyama et a...
Embodiment 2
[0139] This example describes the production of polyclonal antibodies to Arabidopsis ACS.
[0140] PCR primers were designed to allow the coding region of clone J9 to be PCR amplified as a DNA fragment terminated by a restriction site suitable for cloning into the E. coli protein expression plasmid pMALC-2 (New England Biolabs, Beverly, MA). The expression vector clone J9-NT1 / pMALC-2 was constructed according to the instructions provided by the vector supplier. When transcribed into E. coli and induced with isopropylthio-β-galactoside (IPTG), this clone results in the production of a recombinant chimeric fusion protein consisting of the C-terminal portion of the Arabidopsis ACS in conjunction with E. coli maltose protein (MBP) fusion. The recombinant protein was purified from one liter of transformed E. coli culture and purified using amylose columns from New England Biolabs according to the manufacturer's protocol. Purified proteins were analyzed by electrophoresis for puri...
Embodiment 3
[0142] This example describes the cloning of the E1[alpha], E1[beta] and E3 subunit cDNAs of pPDH, and compares the cDNA sequence encoding the E1[beta] subunit with the red algae pPDH sequence and the Arabidopsis mtPDH sequence.
[0143] The deduced amino acid sequences of the E1α and E1β subunits of Porphyra PDH were searched against the dbEST database using the program BLAST. A clone identified as likely to be pPDH was obtained from ABRC at Ohio State University.
[0144] Arabidopsis EST clones 232D14T7 (N65567) and 232D13T7 (N65566) showed a significant degree of homology to the PDH Elα subunit of Porphyra. Both clones contain the same nucleotide sequence, encoding all amino acids except the first 50-70 amino acids of the pPDHE1α subunit. Clone 232D14T7 (D14) was selected for sequencing. The DNA sequence of this clone comprises SEQ ID NO: 3 and the deduced amino acid sequence comprises SEQ ID NO: 4 (see Figure 2 and Sequence Listing).
[0145] Arabidopsis EST clones 163C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com