Glycosylated proteins having reduced allergenicity
A glycosylated protein and allergenic technology, which can be used in peptide/protein components, medical preparations containing active ingredients, applications, etc., and can solve problems such as time-consuming and cost-effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] The following experiments illustrate the reduction of allergenicity of lipase derived from Thermocyces lanuginosus (Humicola langinosa) strain DSM 4109 by means of genetic engineering. Additional glycosylation sites are provided in specific regions of the enzyme. Example 1: Epitope identification and variant selection
[0223] Lipase variants were designed to introduce additional glycosylation sites of the consensus sequence Asn-Xaa-Thr / Ser. To select glycosylation sites, epitopes on wild-type lipase were first identified to maximize macrophage clearance and epitope shielding.
[0224] To identify epitope patterns, phage display libraries expressing random peptide sequences (9-mers) were screened with IgG purified by caprylic acid precipitation from rabbit anti-lipase antiserum. IgG antibodies were immobilized on paramagnetic microspheres carrying specific anti-rabbit IgG antibodies as described by the manufacturer (Sigma). Unbound material was removed, and the coate...
Embodiment 3
[0228] Therefore, lipase variants were expressed in Aspergillus oryzae known to be capable of glycosylation. The secreted lipase variants were confirmed to be glycosylated in cells by comparing the mobility changes of the variants with wild-type in Western blot analysis (attached figure 2 ). Furthermore, the slow migration of the variant (#5) with most glycosylation sites indicated that, as expected, the degree of glycosylation correlated with the number of glycosylation sites inserted into the sequence. Example 3: Keeping functionality of variants
[0229] Wild-type lipase and variants were purified and their specific activities determined. Briefly, a substrate for lipase was prepared by emulsifying tributyrin with gum arabic as an emulsifier. Lipase activity was measured at pH 7 by the pHstat method. One lipase activity unit (LU) is defined as the amount required to release one micromole of fatty acid per minute (Svendsen et al., Methods in Enzymology, vol 284, pp317-34...
Embodiment 4
[0230] Activity was expressed as LU / ml of a solution with the same protein concentration determined by A280 assay. The activity of the wild-type lipase was 4917 LU / ml, the activity of variant #1 was 4478 LU / ml, and the activity of variant #5 was 4222 LU / ml. Therefore, these variants have essentially the same function as wild-type lipase. Example 4: IT-rat experiment (IgG1 / IgE):
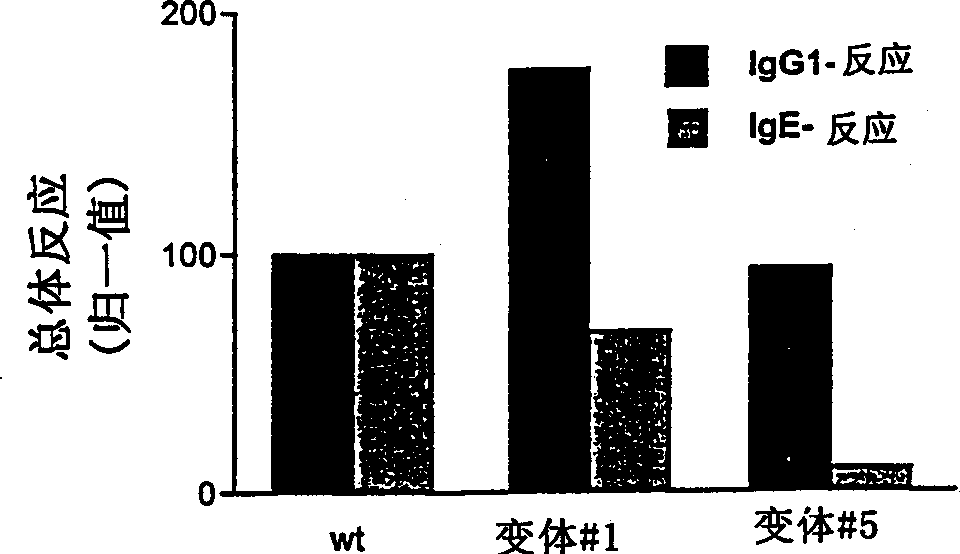
[0231] Antigen and allergen potency of variants #1 and #5 and wild-type lipase were compared in a rat model of intratracheal exposure to antigen. Immunizations: Rats (female Brown-Norway rats with an average weight of 180 g) were immunized 20 times intratracheally with 100 μl of 0.9% (wt / vol) NaCl (control group) or 100 μl of saline solution containing 15 μg of protein. Group 1 received wild-type lipase, group 2 received lipase variant #1, and group 3 received lipase variant #5. Each group consisted of 10 rats. Blood samples (2 ml) were collected from the eye 1 week after each booster immunization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com