Technological process of continuously perfused culture of hybrid tumor and CHO cell to prepare antibody
A process method and hybridoma technology, applied in the field of bioengineering, can solve the problems of low adsorption efficiency and short life of monoclonal antibody, and achieve ideal effect, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
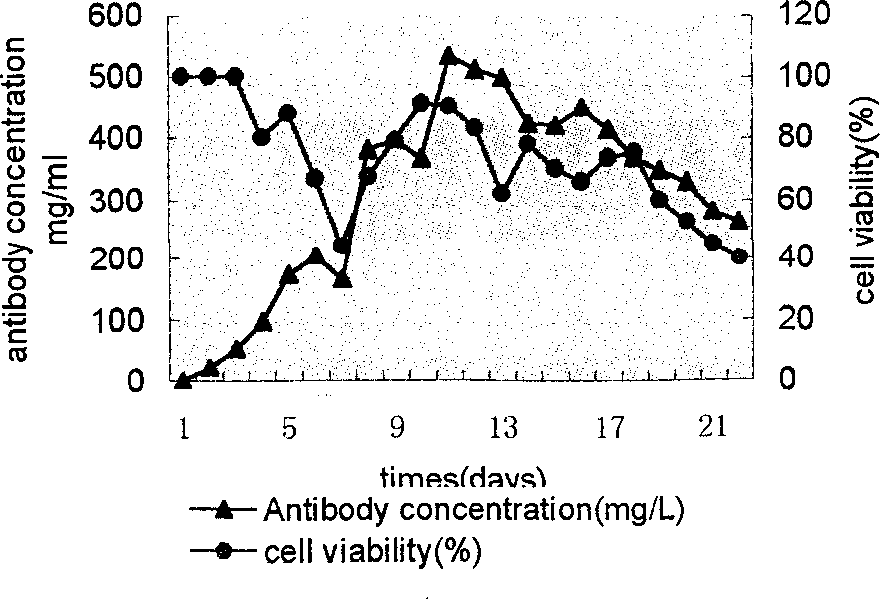
Image
Examples
Embodiment Construction
[0013] The target product of the present invention is a mouse-derived monoclonal antibody secreted by hybridoma cells (HAb18). 31 I-HAb18 F(ab') 2 Hepatocarcinoma monoclonal antibody injection has entered Phase II and Phase III clinical trials. Liver cancer monoclonal antibody HAb18 IgG1 and F(ab') 2 Fragment antibodies have high specificity and affinity for primary hepatocellular carcinoma. The preparation process and technical route of the present invention can be applied to pilot-scale research and large-scale production of monoclonal antibodies and humanized antibodies. The steps are as follows:
[0014] 1. Large-scale culture of hybridoma cells (HAb18):
[0015] 1. Preparation of seed cells
[0016] (1) Expansion of seed cells
[0017]HAB18 hybridoma cells were prepared by immunizing BABL / c mice with the cell suspension of fresh liver cancer surgical specimens and fused the splenocytes with mouse myeloma SP / 20 cells. Established in August 1987, it has been stably s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com