Gene therapeutics
A gene therapy and functional technology, applied in gene therapy, biochemical equipment and methods, double-stranded DNA virus, etc., can solve problems such as failure to achieve guiding effect, low efficiency, and damage to infection function combined with function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (1) The vector PGK-hADA ("Natural Medicine" 2: 876-882 (1996))-containing human ADA produced by EPHA-5-producer cells ("Natural Medicine" 2: 876-882 (1996)) The PGK vector of the gene (hADA), and the injection preparation of RetroNectin as described in Example 2 below were injected into the tail vein of C3H / HeJ mice (8 weeks old, purchased from Japan SLC). Transduction of hematopoietic stem cells was analyzed by examining the expression of transduced human ADA cDNA in mice bearing the transgene as described in Nature Medicine 2:876-882 (1996). Specifically, the presence of human ADA protein in peripheral blood cells derived from mice was confirmed by ADA isozyme analysis using cellulose acetate electrophoresis for detection. Exams are performed at the beginning of the fourth month after transplantation and repeated monthly.
[0079] (2) For mice administered with RetroNectin and the vector PGK-hADA, expression of human ADA cDNA was confirmed by analysis of transduced b...
Embodiment 2
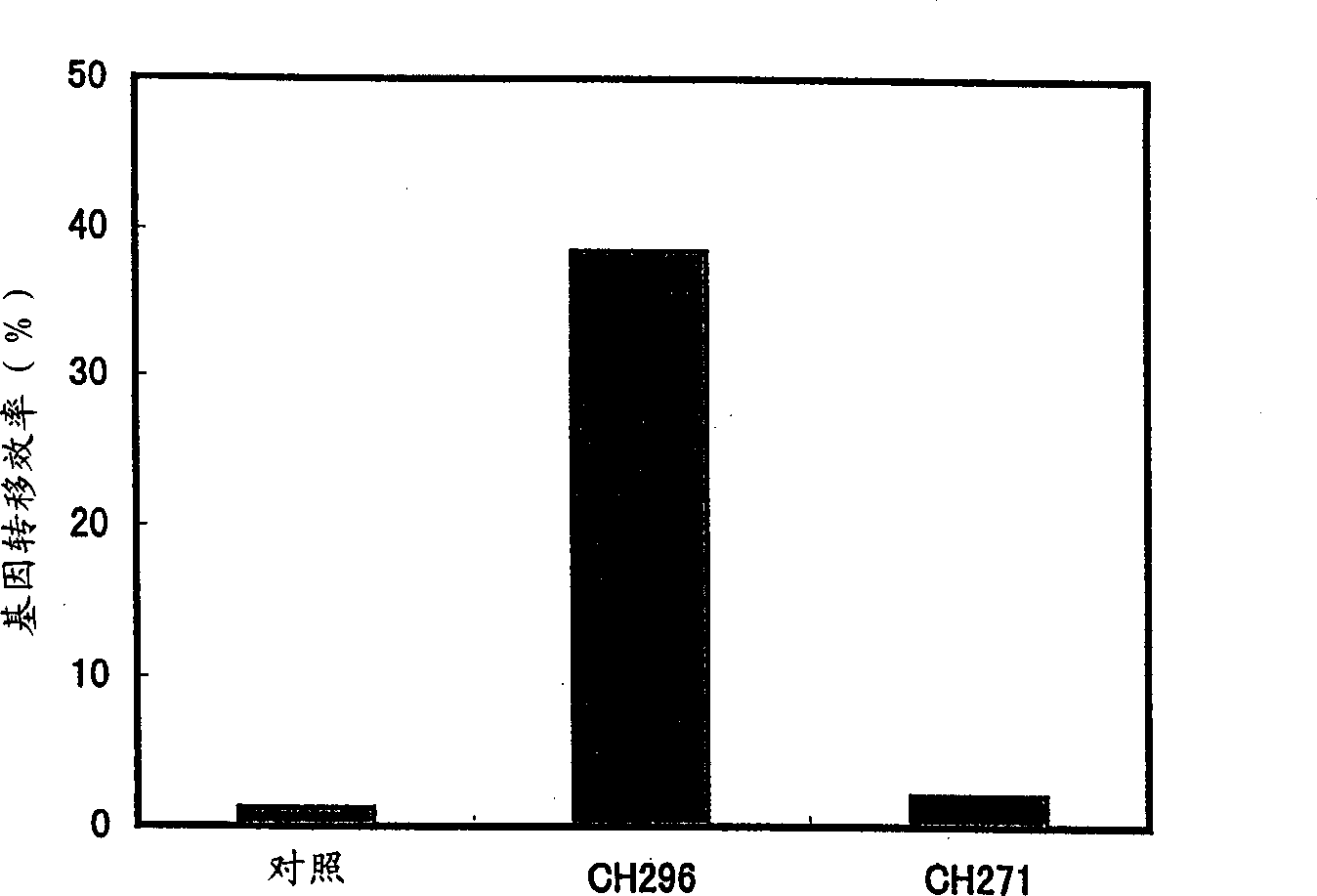
[0081] RetroNectin (CH-296, Takara Shuzo) was dissolved in water for injection at a concentration of 2 mg / ml. This solution was equilibrated with saline to prepare a formulation for injection.
Embodiment 3
[0082] Example 3: Gene transfer using HL-60 cells as vectors
[0083] Polypeptide CH-271 was prepared as described below. Briefly, Escherichia coli HB101 / pCH101 (FERM BP-2799) was cultured according to the method described in US Patent No. 5,198,423. CH-271 was obtained from the culture.
[0084] Human leukemia HL-60 cells (purchased from Dainippon Pharmaceutical) were used at 2×10 6 Cells / ml were suspended in D-EME medium (Bio Whittaker) containing 10% fetal calf serum (FCS, Bio Whittaker). RetroNectin TM (Takara Shuzo) or CH-271 was added to 1 ml of the cell suspension at a final concentration of 100 μg / ml. A control group to which no such functional substance was added was provided.
[0085] 100 μl containing 6.23 × 10 6 cfu / ml A solution of an environmental retroviral vector with an enhanced green fluorescent protein (EGFP) gene (pLELN (Clontech), prepared using GP+E-86 cells (ATCC CRL-9642)) was added to the cells. The mixture was incubated at 37°C for 30 minutes i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com