Preparation absorbed through skin
A preparation and adhesive technology, which is applied in the field of percutaneous absorption preparations, can solve problems such as skin irritation, and achieve the effect of less skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102]An acrylic adhesive (75 parts, (2-ethylhexyl) acrylate / acrylic acid copolymer, (2-ethylhexyl) acrylate / acrylic acid=95 / 5 (weight ratio)) was dissolved in ethyl acetate. Diethyl sebacate (20 parts) and free-form ethylpyridine (5 parts) were added thereto to obtain an adhesive layer-forming coating solution. This coating solution was applied onto a release-treated polyester film to a thickness of 30 µm after drying, and dried (formation of an adhesive layer B). Separately, the above coating solution was coated on a carrier non-woven fabric (polyester film with a thickness of 6 μm and a basis weight of 8 g / m 2 on a laminate of polyester nonwoven fabric) to a thickness of 60 μm after drying, and dried (to form an adhesive layer A).
[0103] As the release controlling layer, a 50 m thick high molecular weight porous polyethylene film (porosity: 45%, polyethylene film produced by NITTO DENKO CORPORATION, trademark BREATHRON) was used. Adhesive layer B on the polyester film o...
Embodiment 2
[0105] A polyisobutylene binder (60 parts, a mixture of polyisobutylene having a viscosity average molecular weight of 1,400,000 (100 parts) and polyisobutylene having a viscosity average molecular weight of 60,000 (100 parts)) was dissolved in hexane. Isopropyl myristate (30 parts) and free-form ethylpyridine (10 parts) were added to obtain a coating solution for forming an adhesive layer. This coating solution was applied onto a release-treated polyester film to a thickness of 20 µm after drying, and dried (formation of an adhesive layer B). Separately, the above coating solution was coated on a carrier non-woven fabric (polyester film with a thickness of 6 μm and a basis weight of 8 g / m 2 on a laminate of polyester nonwoven fabric) to a thickness of 30 micrometers after drying, and dried (to form an adhesive layer A).
[0106] As the release controlling layer, a 50 µm thick ultrahigh molecular weight porous polyethylene film (porosity: 30%, ultrahigh molecular weight polye...
Embodiment 1
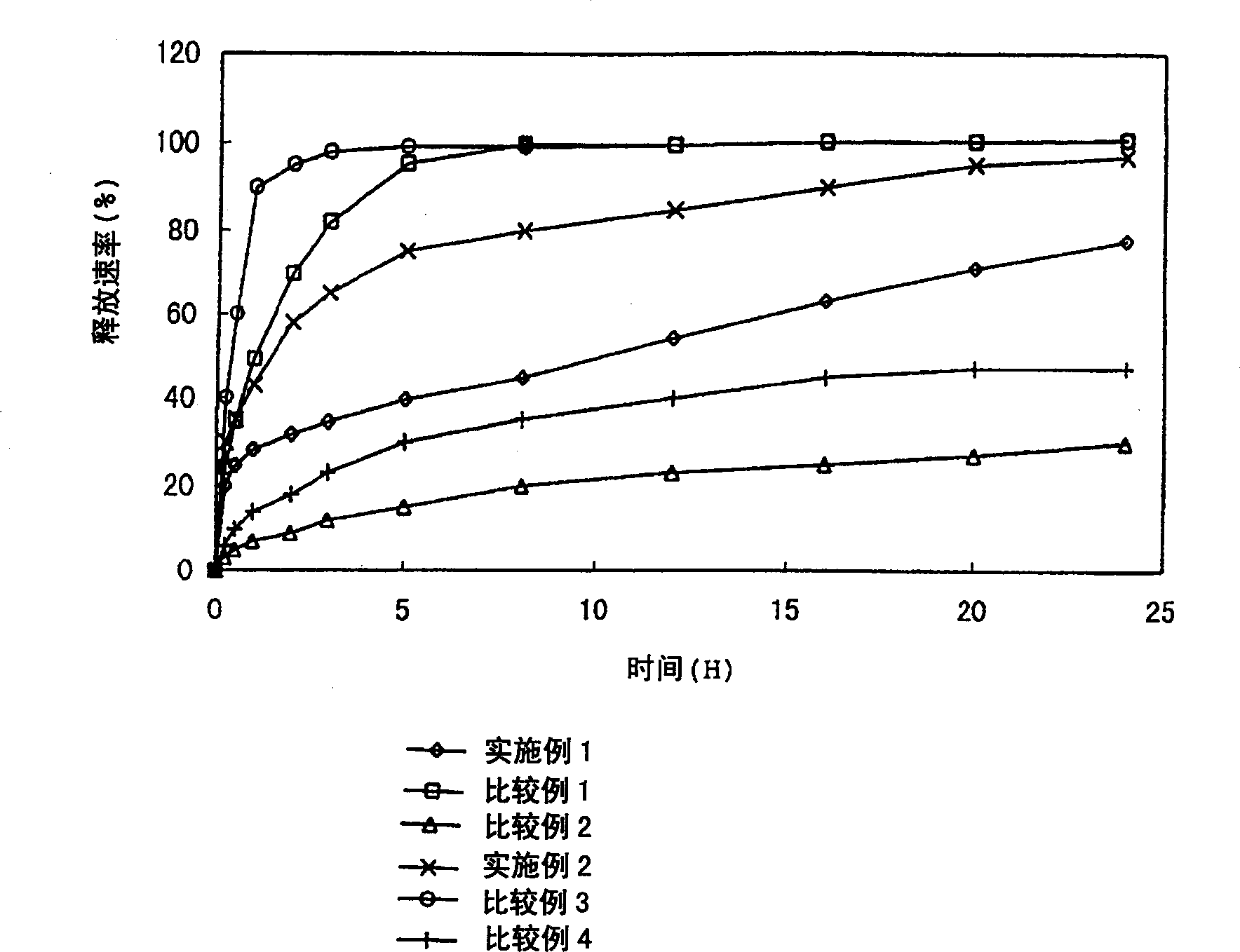
[0112] The percutaneous absorption preparations obtained in the above-mentioned examples and comparative examples were punched into 10 cm 2 Rounds, and subjected to underwater release tests according to Japanese Pharmacopoeia, Diffusion Test Method 2 (Pump Method). The results are shown in figure 1 . figure 1 The results clearly show that the presence of the release controlling layer in the adhesive layer can give the desired controlled pattern of drug release. Test Example 2 (skin irritation test)
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com