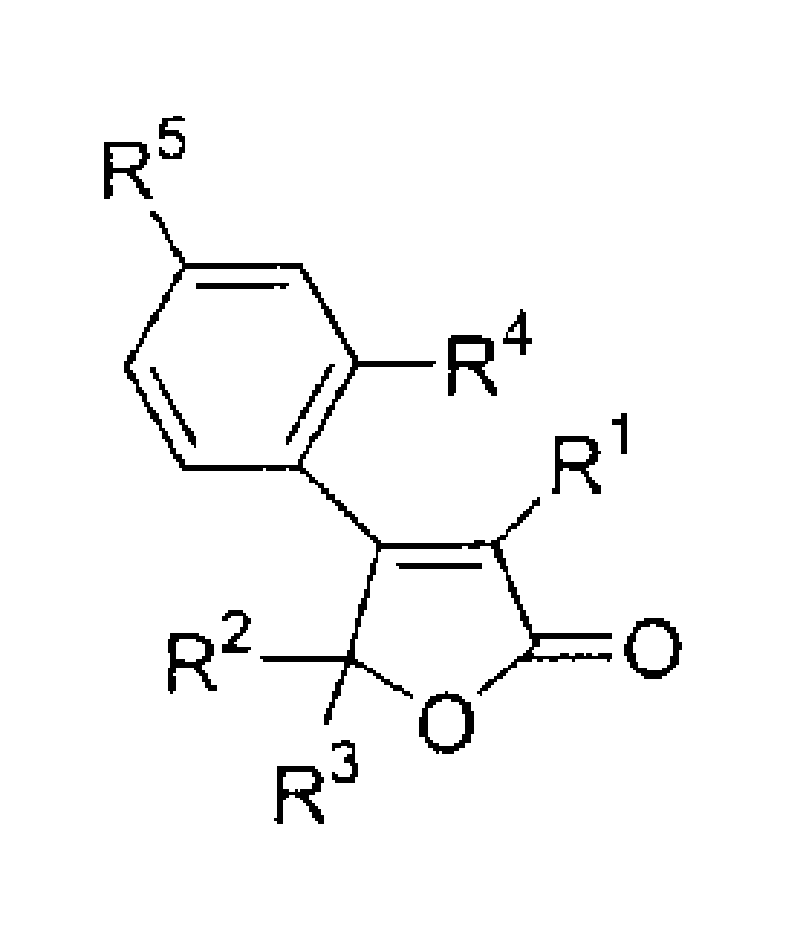
Gamma-crotonic lactone with substituted aryl group in its 4th place and its solid phase synthesis process
A solid-phase synthesis method and a technology of butenolide, which is applied in the field of five-membered ring unsaturated lactone and its synthesis, can solve the problems of unsatisfactory yield and time-consuming, and achieve easy automation, low cost and good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1.851 grams of functionalized polystyrene resin (theoretical maximum loading capacity 2.5 meq / g) was added to 55 milliliters of THF, under stirring at room temperature, 2.206 grams of p-iodobenzyl alcohol and 0.330 grams of sodium hydride were added successively, and the mixture was stirred Reflux for three days. Then cooled to room temperature, filtered, followed by DMF (10 ml × 3), DMF / H 2 O (10ml×3), H 2 The resin was washed with O (10 mL×3), DMF (10 mL×2), MeOH (20 mL×3), and dried in vacuo overnight to obtain 2.590 g of p-iodobenzyl ether supported on the resin.
[0027] 246 mg of the resin prepared by the above steps (theoretical maximum loading capacity 1.674 meq / g) was added to 2 ml of anhydrous acetonitrile, and 292 mg of 2,3-alkenedecanoic acid, diiso 0.56 ml of propylethylamine, 70 mg of tetrakis(triphenylphosphine) palladium and another 2 ml of anhydrous acetonitrile were reacted at 70°C for three days. Then cooled to room temperature, filtered, followed ...
Embodiment 2
[0030] 1.007 grams of functionalized polystyrene resin (theoretical maximum loading capacity 2.5 millieq / g) was added to 30 milliliters of THF, under stirring at room temperature, 1.200 grams of o-iodobenzyl alcohol and 0.180 grams of sodium hydride were added successively, and the mixture was stirred Reflux for three days. Then cooled to room temperature, filtered, followed by DMF (10 ml × 3), DMF / H 2 O (10ml×3), H 2 The resin was washed with O (10 mL×3), DMF (10 mL×2), MeOH (20 mL×3), and dried in vacuo overnight to obtain 1.385 g of o-iodobenzyl ether supported on the resin.
[0031] 93 mg of the resin (theoretical maximum loading 1.674 meq / g) prepared by the above steps was added to 2 ml of anhydrous acetonitrile, and 83 mg of 2,3-linked diene octanoic acid, diisopropyl 0.21 ml of ethyl ethylamine, 26 mg of tetrakis(triphenylphosphine) palladium and another 2 ml of anhydrous acetonitrile were reacted at 70° C. for three days. Then cooled to room temperature, filtered, f...
Embodiment 3
[0034] 92 mg of p-iodobenzyl ether loaded on the resin of Example 1 was added to 2 ml of anhydrous acetonitrile, and 84 mg of 2,3-diene octanoic acid and 0.21 ml of diisopropylethylamine were added successively under the protection of argon. React with 26 mg of tetrakis(triphenylphosphine) palladium and another 2 ml of anhydrous acetonitrile at 70° C. for three days. Then cooled to room temperature, filtered, followed by DMF (5 ml × 3), DMF / H 2 O (5ml×3), H 2 The resin was washed with O (5 mL×3), DMF (5 mL×2), MeOH (10 mL×3), and dried in vacuo overnight to obtain 99 mg of γ-butenolide loaded on the resin.
[0035] To a solution of 95 mg of the resin prepared above in dichloromethane (4 mL), add ZnBr 2 (24 mg) and bromoacetyl (0.33 ml). It was then stirred at room temperature for 24 hours and filtered. The filtrate was transferred to a separatory funnel, washed successively with 5% sodium bicarbonate solution, 5% hydrochloric acid solution and saturated brine, dried, and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com