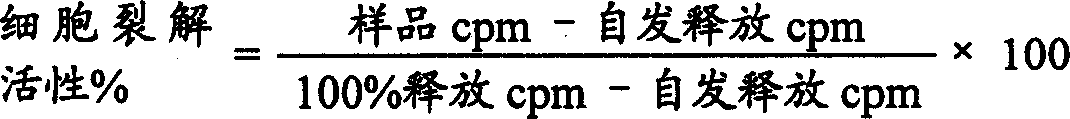Method for inhibiting macrophage infiltration using monoconal anti-alpha-D-antibodies
A monoclonal antibody, macrophage technology, applied in other fields, can solve the problem of indistinguishable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Try to detect canine alpha TM1 homologue of
[0056] In an identification dog alpha TM1 In an attempt of the human homologue, the test was performed against the canine α TM1 Cross-reactivity of the specific monoclonal antibody Ca11.8H2 [Moore et al., supra] on human peripheral blood leukocytes. In the presence of 0.1% sodium azide, cell preparations (usually 1×10 6 cells) were incubated on ice with undiluted hybridoma supernatant or purified mouse IgG negative control antibody (10 μg / ml). Binding of monoclonal antibodies was detected by subsequent incubation with 6 μg / ml FITC-conjugated horse anti-mouse IgG (Vector Laboratories, Burlingame, CA). Stained cells were fixed with 2% w / v paraformaldehyde in phosphate buffered saline (PBS) and analyzed with a Facstar Plus fluorescence activated cell sorter (Becton Dickinson, Mountain View, CA). Typically, 10,000 cells are analyzed with logarithmic amplification of fluorescence intensity.
[0057] The result...
Embodiment 2
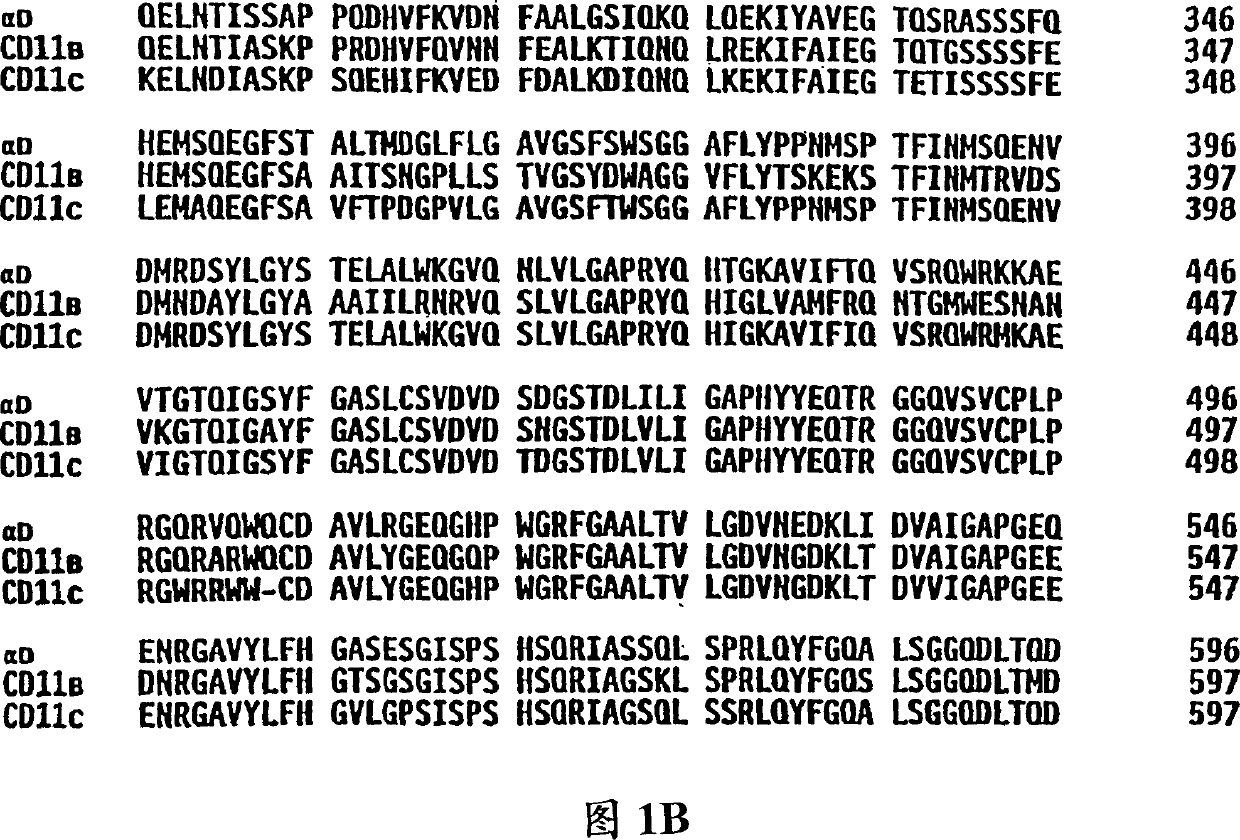
[0060] Affinity purified canine alpha TM1 for N-terminal sequencing
[0061] Affinity purified canine alpha TM1 To determine the N-terminal amino acid sequence for the design of oligonucleotide probes / primers. In short, the anti-alpha TM1 Monoclonal antibody Ca11.8H2 with Affigel 10 Chromatography resin (BioRad, Hercules, CA) coupled to separate proteins by specific antibody-protein interactions. Antibodies were conjugated to the resin at a concentration of approximately 5 mg antibody / ml resin following the protocol suggested by BioRad. After the conjugation reaction, excess antibody was removed and the resin was blocked with three volumes of 0.1 M ethanolamine. The resin was then washed with 30 column volumes of phosphate buffered saline (PBS).
[0062] 25 grams from a single dog spleen 23 grams of a single dog spleen was homogenized in 250 ml of 25 mM Tris-HCl pH 8.0 buffer containing 0.32 M sucrose and protease inhibitors. Nucleic acids and cellular ...
Embodiment 3
[0077] Large scale affinity purification of canine alpha TM1 for in-house sequencing
[0078] To provide additional amino acid sequences for primer design, purified canine α TM1 For in-house sequencing. Three frozen spleen sections (about 50 g each) and frozen cells from two partial spleens from adult dogs were used to generate proteins for in-house sequencing. 50 g of spleen was homogenized in 200-300 ml of boric acid buffer with a Waring blender. The homogenized material was diluted with 1 volume of buffer containing 4% NP-40, and the mixture was stirred gently for at least 1 hour. Large debris in the resulting lysate was removed by centrifugation at 200Og for 20 minutes, then filtered through either a Corning (Corning, NY) prefilter or a Corning 0.8 micron filter. The lysate was further clarified by passing through a Corning 0.4 micron filter system.
[0079] Spleen lysate and antibody-conjugated Affigel as described in Example 2 10 Resins were mixed in 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com