Sulfoalkyl ether cyclodextrin based controlled release solid pharmaceutical formulations
A technology of solid pharmaceutical preparations and sulfoalkyl ethers, which is applied in the field of controlled-release solid pharmaceutical preparations and can solve problems such as the complexity of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
[0146] Example 8 details a method of preparing an exemplary embodiment of a multilayer tablet according to the present invention comprising at least one immediate release layer adjacent to a controlled release layer. Figure 23a Depicted is a bilayer tablet (1) comprising an immediate release layer (3) containing the major amount of drug and SAE-CD present together in a physical mixture, and a controlled release layer (2), and an immediate release layer (3) The matrix, while the controlled release layer contains a physical mixture of the main amount of drug, SAE-CD and release rate modifier. The immediate release layer disintegrates and releases the drug in the environment immediately after administration to the patient or addition of the tablet to the dissolution medium. Figure 23b depicts a three-layer tablet in which a controlled release layer (6) prepared as in Example 8 is sandwiched between two rapid release layers (5a, 5b) prepared as described in Example 8. It should ...
Embodiment 11
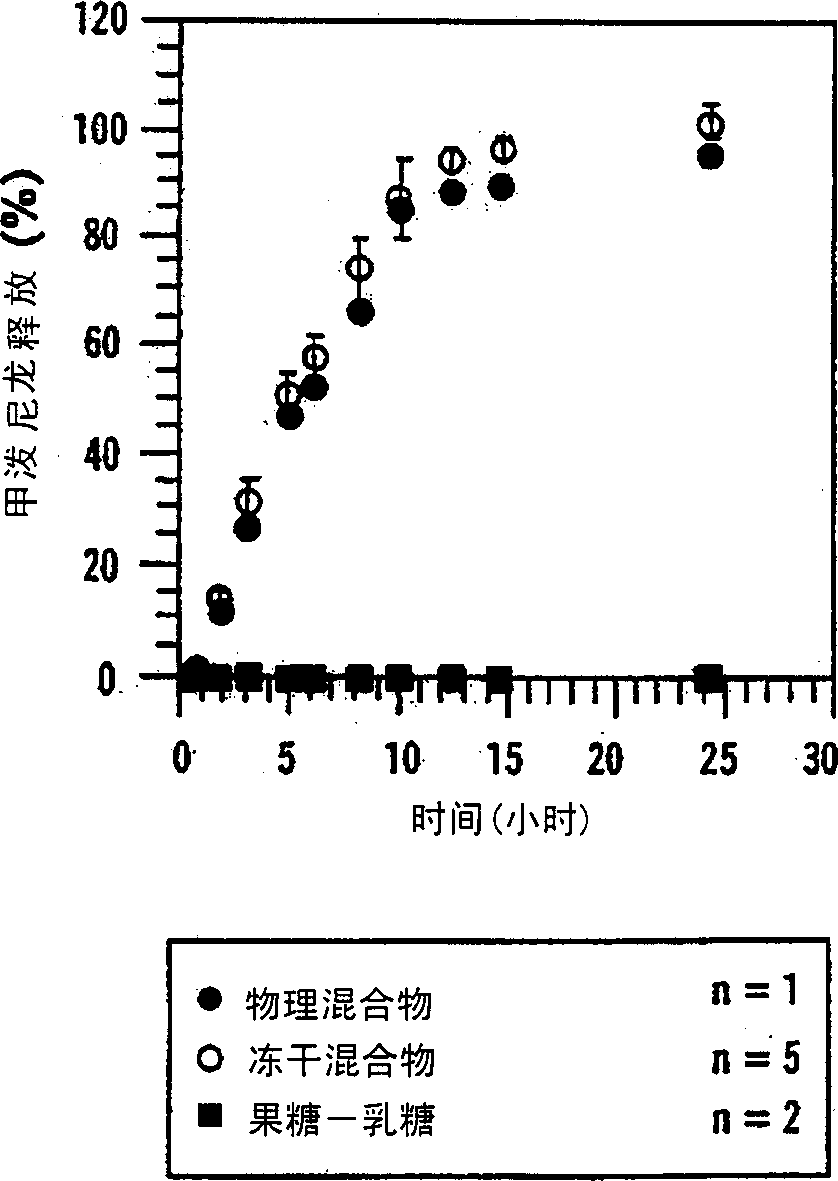
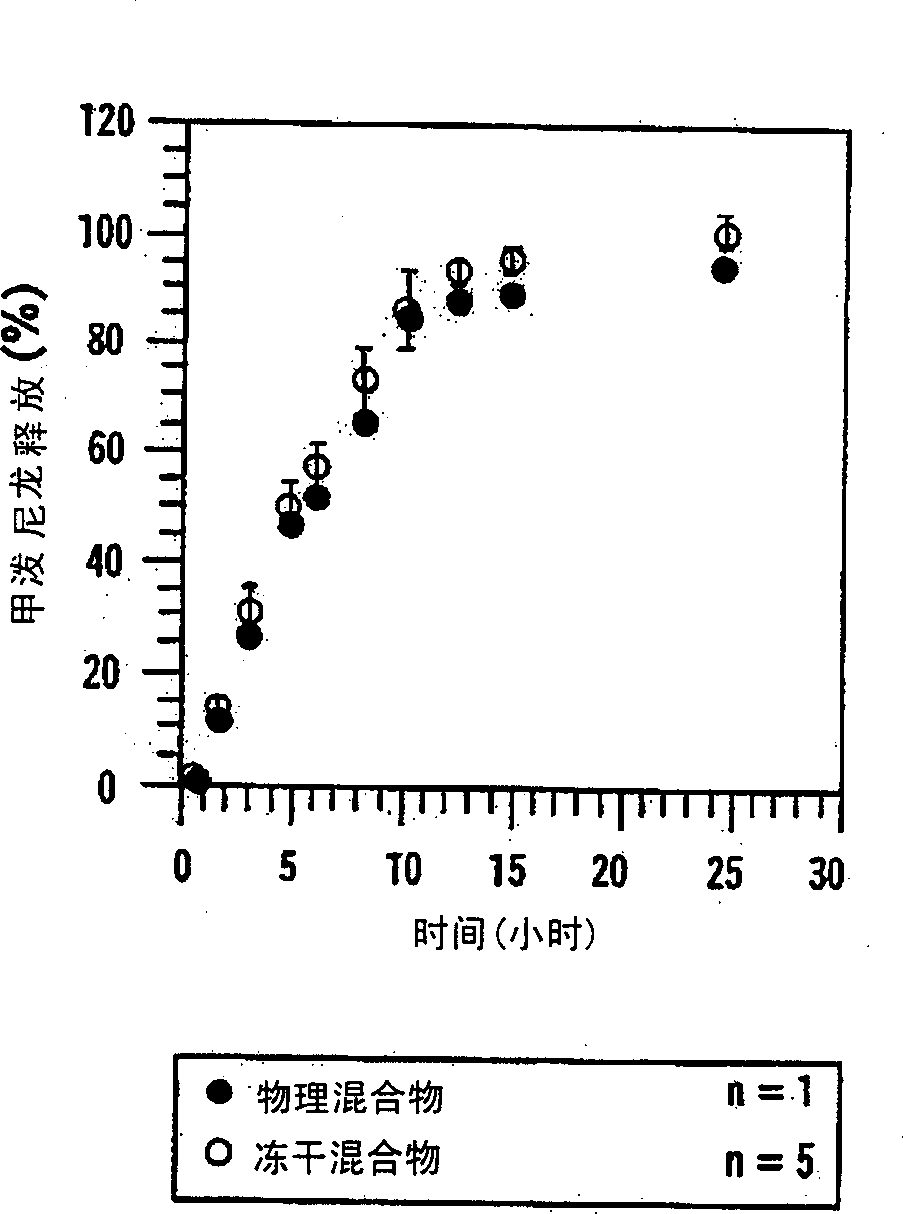
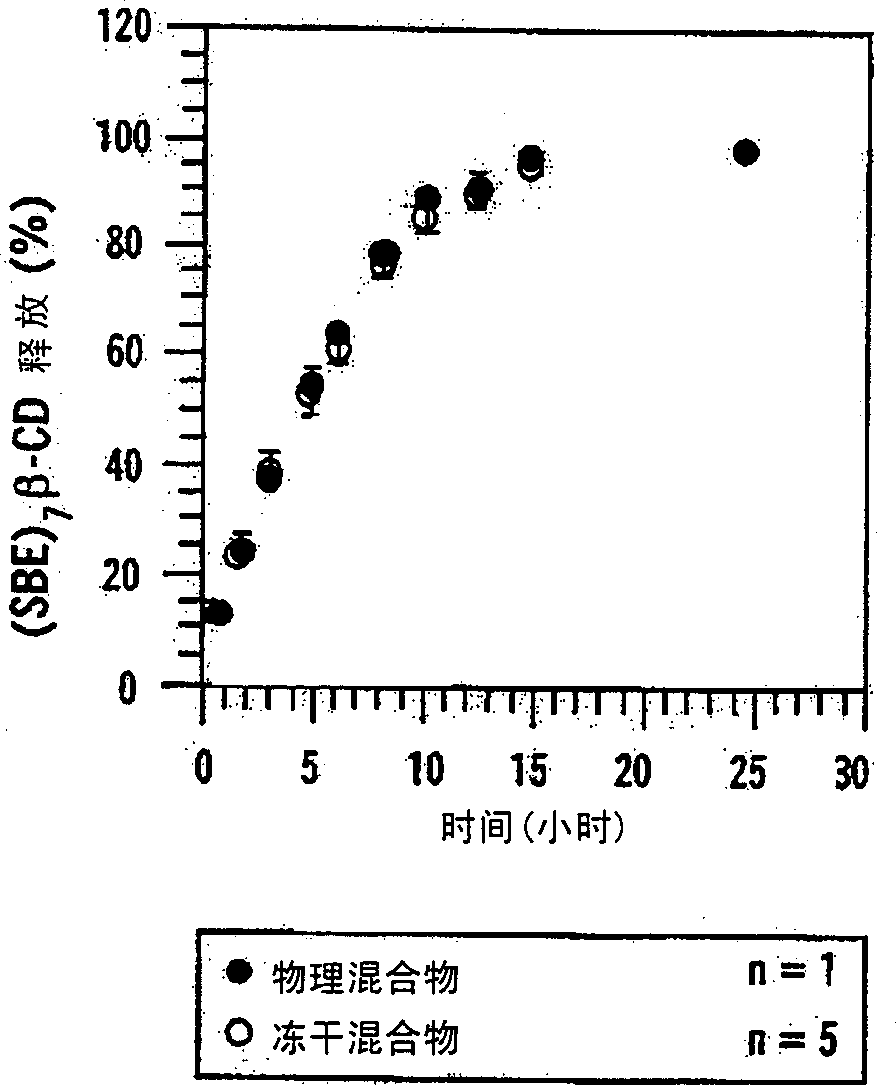
[0153] Example 11 describes the preparation of an osmotic pump that releases testosterone (TS) into an environment of use through a combination of diffusion and osmosis. Figure 25 TS, sugar, hydroxypropyl-β-cyclodextrin (HP-β-CD) and (SBE) are depicted 7m - Comparative profiles of β-CD release from various osmotic pump formulations. The first formulation contained a physical mixture of TS(○) and a sugar mixture (●, lactose and fructose, 1:1); the second formulation contained TS(□) and (SBE) 7m -β-CD (■); while the third formulation contained TS (◇) and HP-β-CD (◆). The results showed that, compared with osmotic pumps containing sugar mixture or HP-β-CD, containing (SBE) 7m -Osmotic pumps for β-CD released greater amounts of TS at a more acceptable rate.
[0154] The advantageous properties of the present formulation allow the preparation of drug release devices with combined and controlled diffusion and osmotic release of drug. These devices can be prepared by varying the...
Embodiment 1
[0218] Testosterone-(SBE) 7 -β-CD sustained release formulation
[0219] This example demonstrates the application of the present invention in the preparation of sustained-release preparations, wherein the pharmacologically active agent is testosterone as an example. Phase Solubility Studies
[0220] Add excess testosterone in 0.25ml of (SBE) 7 - In β-CD solution, the concentration range is 0.0-0.05 mmol / l. The dispersion was equilibrated for at least 24 hours in a shaking water bath (100 rpm, 25°C). The dispersion was centrifuged at 2500 rpm for 10 minutes, and 20 μl of the supernatant was sampled with an airtight 100 μl syringe (Hamilton Co., NV), diluted with mobile phase, and then analyzed for testosterone concentration in the solution by HPLC. With Higuchi and Connors for A L Testosterone-(SBE) by Type Diagram Method 7 -β-CD binding constant K 1∶1 . Preparation of tablet cores
[0221] With a 1:1 molar ratio of testosterone / (SBE) 7 - β-CD Preparation of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com