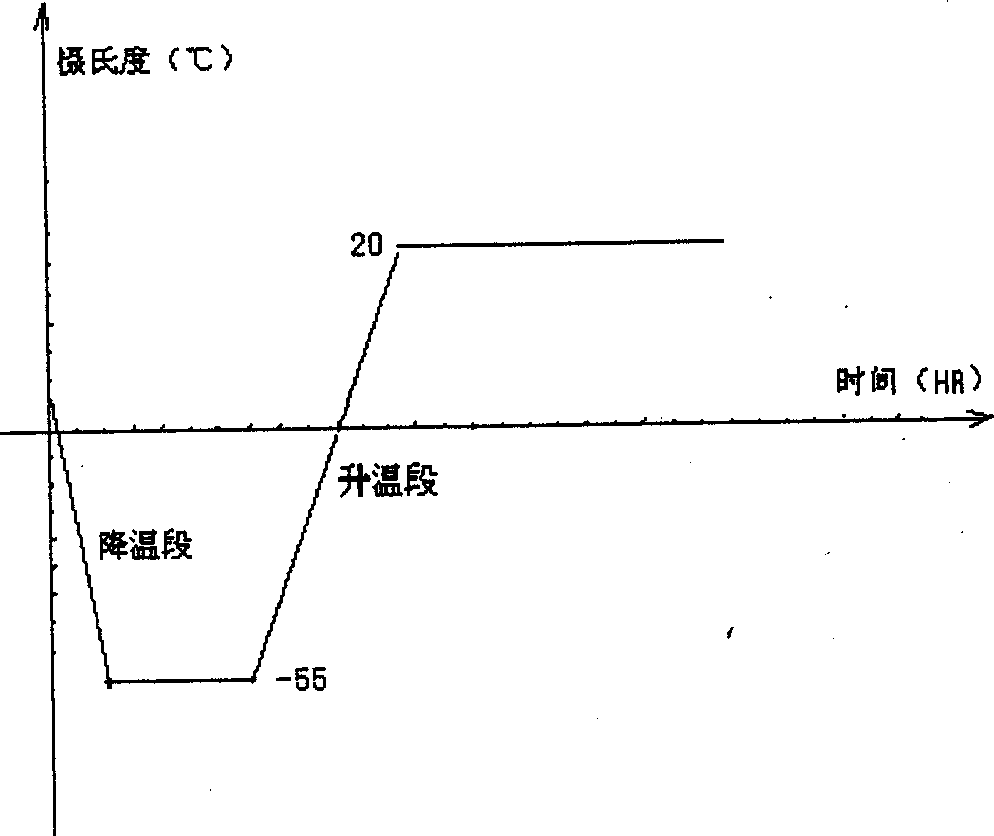
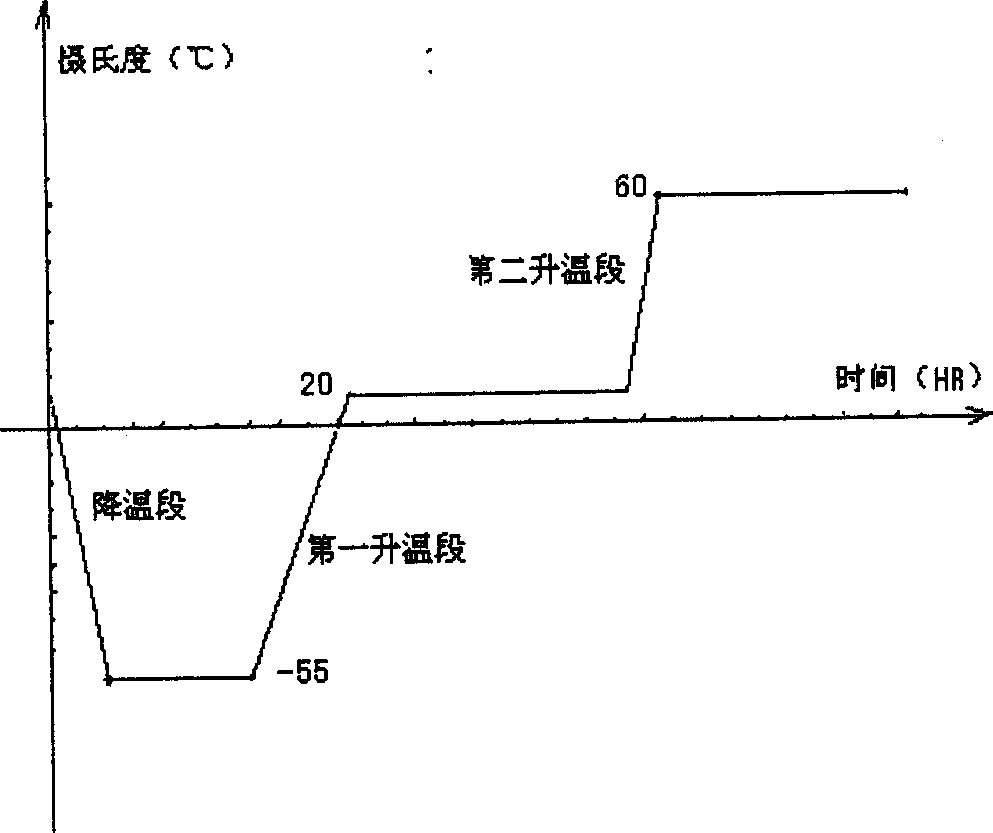
Freeze dried hydroxy camptothecin powder for injection and its preparing process
A technology of hydroxycamptothecin and freeze-dried powder injection, which is applied in the direction of freeze-dried transportation, medical preparations containing active ingredients, powder transportation, etc., which can solve the impact of safety and effectiveness, large differences between batches, and limited stability In order to achieve the effects of convenient storage, improved stability and extended validity period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
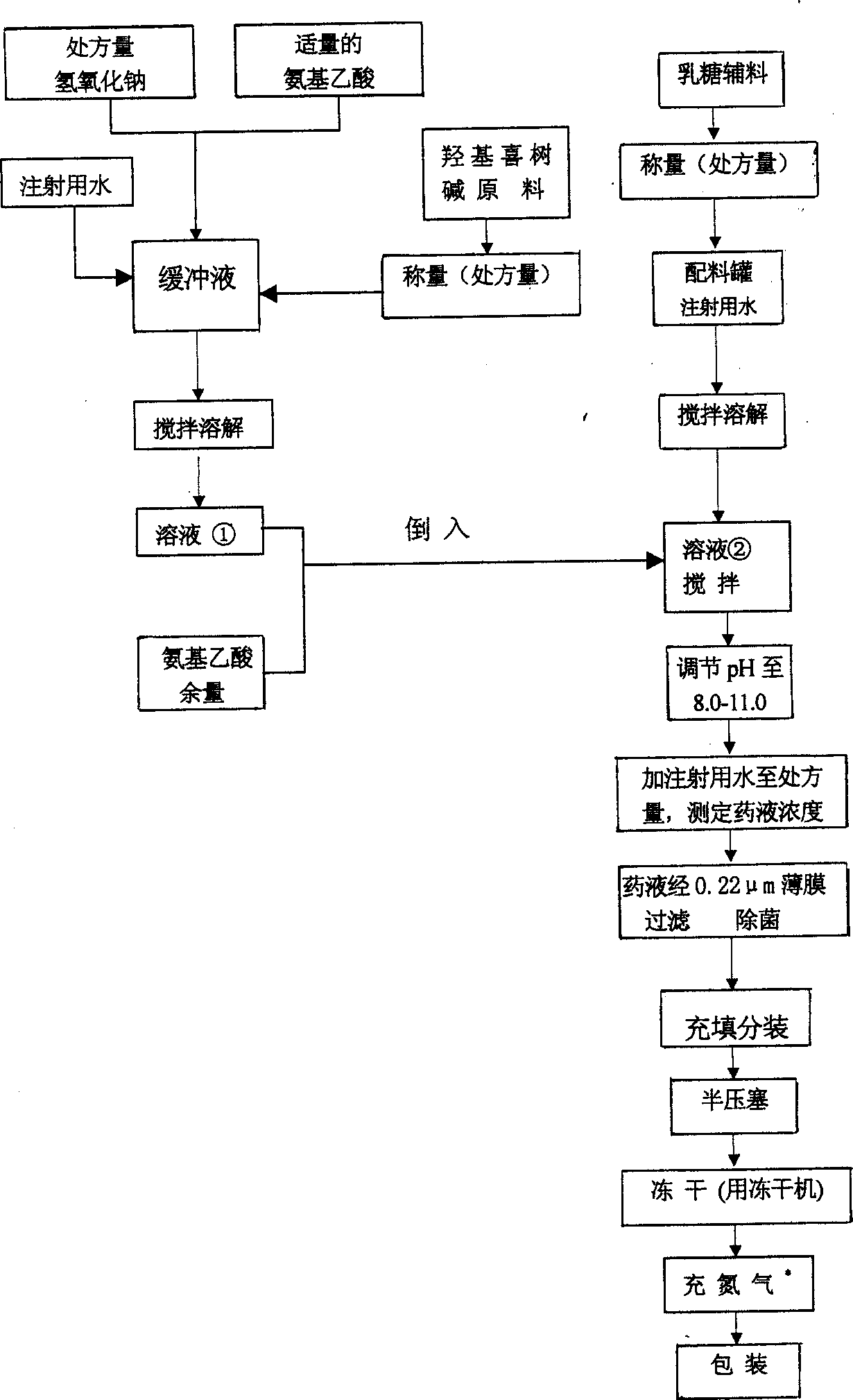
[0023] Embodiment 1: 2mg / vial specification 1000 freeze-dried powder preparation formulations are:
[0024] Hydroxycamptothecin 1.8g, Glycine 2.2g,
[0025] Lactose 45.0g, Sodium Hydroxide 10.0g,
[0026] Add water for injection to 2100ml, make 1000 altogether.
[0027] Wherein, hydroxycamptothecin is the main drug, and feeds are fed respectively according to the requirements of two kinds of production specifications (2mg / branch, 5mg / branch).
[0028] Lactose is an excipient, used as a skeleton support part of hydroxycamptothecin freeze-dried powder for injection. According to the literature reports and the practical experience of other anti-tumor freeze-dried preparations of our company, the test was carried out by simulating the actual production, and the ratio of the main drug to lactose was determined: for the products of 2mg and 5mg specifications, the ratio of 1:20-120 was used. Proportion.
[0029] The buffer solution is made up of aminoacetic acid and sodium hydrox...
Embodiment 2
[0043] Embodiment 2: 2mg / vial specification 1000 freeze-dried powder preparation formulations are:
[0044] Hydroxycamptothecin 2.0g, Glycine 4.0g,
[0045]Lactose 100.0g, Sodium Hydroxide 10.0g,
[0046] Add water for injection to 2500ml, make 1000 altogether.
[0047] Its preparation process is as follows:
[0048] a', according to embodiment 2 preparation prescription batching, get the glycine of sodium hydroxide 10g, 3g (recipe quantity is 4.0g) and the water for injection of 100ml to prepare buffer, buffer is alkaline, pH is 8.5-10.0, Among them, the concentration of aminoacetic acid is 1mol / L, and the concentration of sodium hydroxide is 1% (W / L).
[0049] b', hydroxycamptothecin 2.0g is added in the buffer solution, stirred and dissolved to obtain solution ①;
[0050] c', put 100.0g of lactose into the batching tank with 200ml of water for injection, the water temperature is 20°C, stir and dissolve to obtain a solution②;
[0051] d', Pour the solution ① and the rem...
Embodiment 3
[0059] Example 3: 1000 freeze-dried powder injection formulations with a specification of 5 mg / vial are composed as follows:
[0060] Hydroxycamptothecin 5.0g, Glycine 8.0g,
[0061] Lactose 300.0g, sodium hydroxide 10.0g,
[0062] Add water for injection to 4000ml, make 1000 altogether.
[0063] Its preparation process is as follows:
[0064] a ", according to embodiment 3 preparation prescription batching, get the glycine of sodium hydroxide 10g, 5.6g (recipe quantity is 8.0g) and the water for injection of 300ml to prepare buffer, buffer is alkaline, pH is 7.5-10.5 , wherein the glycine concentration is 1mol / L, and the sodium hydroxide concentration is 1% (W / L).
[0065] b", adding 5.0 g of hydroxycamptothecin to the buffer solution, stirring and dissolving to obtain a solution ①;
[0066] c", put 300.0g of lactose into a batching tank filled with 300ml of water for injection, the water temperature is 25°C, stir and dissolve to obtain a solution②;
[0067] d", Pour the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com