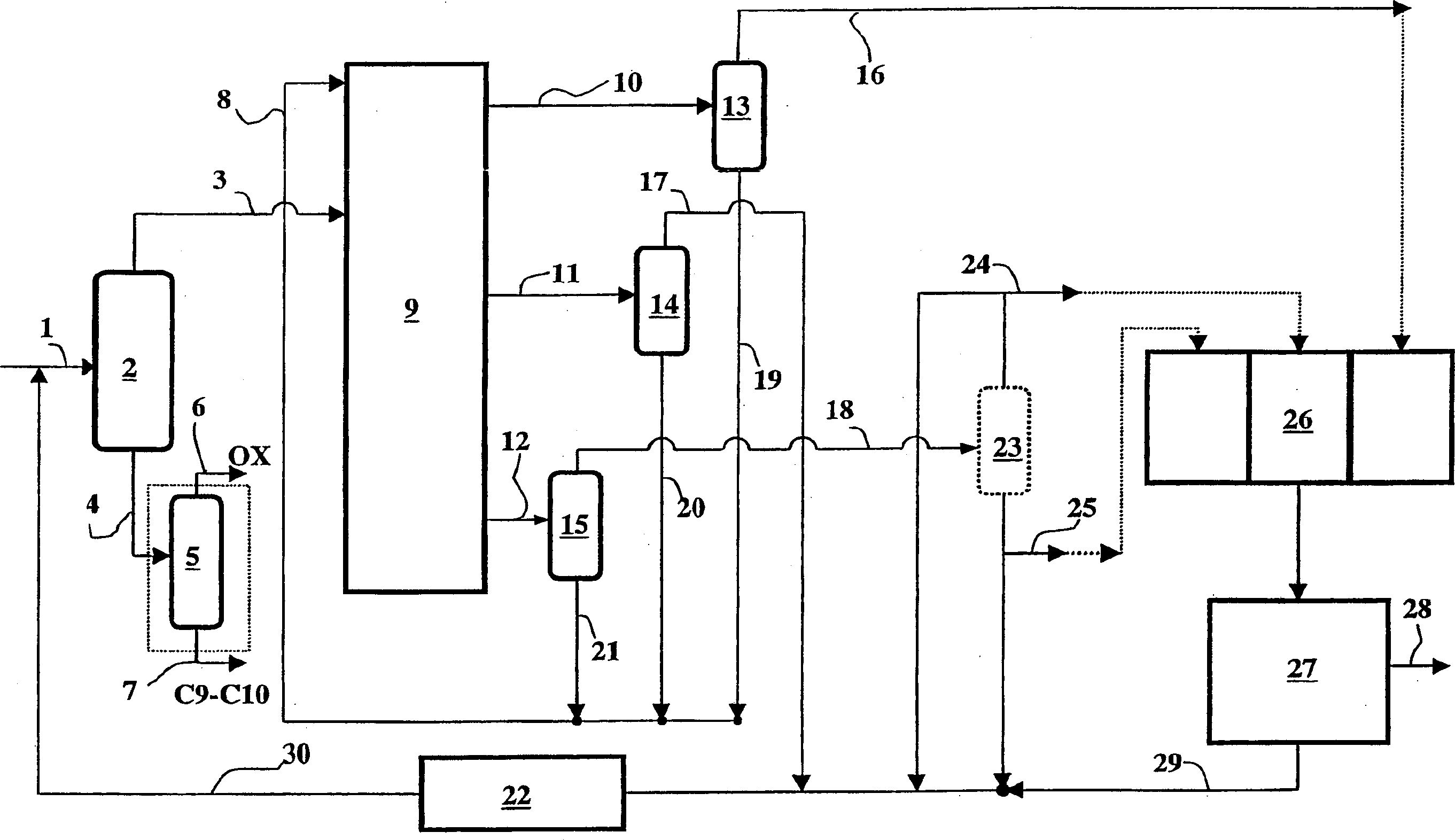
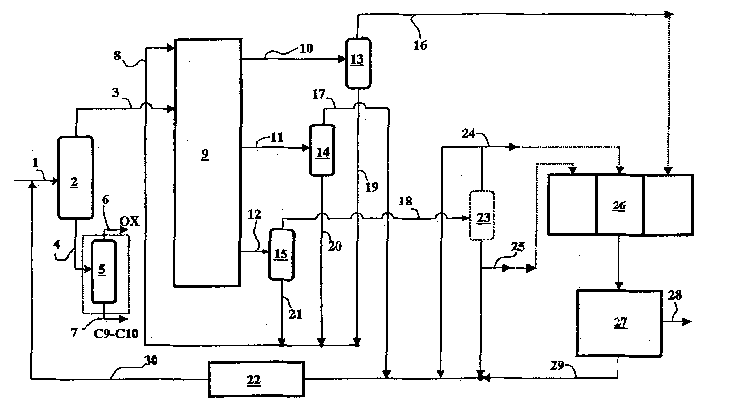
Co-production method for dimethyl benzene, meta dimethyl benzene and/or ortho dimethyl benzene
A technology of o-xylene and m-xylene, applied in chemical instruments and methods, distillation purification/separation, adsorption purification/separation, etc., can solve problems such as increasing flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Using a material containing a mixture of xylene and ethylbenzene, which has previously removed hydrocarbons containing 9-10 carbon atoms, a countercurrent simulated moving bed is used to produce p-xylene, and the material has the following weight composition:
[0067] EB: Ethylbenzene 5.6%
[0068] PX: Paraxylene 226%
[0069] MX: m-xylene 49.9%
[0070] OX: o-xylene 21.9%.
[0071] The pilot plant used in this example consisted of 24 beds 1.1 meters long and 0.021 meters in diameter. Each column was loaded with 344 grams of barium-exchanged X zeolite with a moisture content of 5.5% expressed as loss on ignition at 900°C. The operating temperature is 175°C, the suction pressure of the circulating pump is maintained at 10 bar, all streams are continuously injected or discharged under flow control, except that the intermediate raffinate is continuously discharged under pressure control, and the flow rates of injection and discharge are all at Expressed under ambient p...
Embodiment 2
[0091] Repeat the operation of Example 1, adding an o-xylene distillation tower before the crystallization section, so as to improve the crystallization yield.
[0092] As before, 1.2 liters / hour of raffinate R2 is distilled to obtain a fluid flow rate of 0.13 liters / hour, and its weight composition is as follows:
[0093] EB: Ethylbenzene 0.1%
[0094] PX: p-xylene 1.7%
[0095] MX: m-xylene 73.9%
[0096] OX: o-xylene 24.2%.
[0097] The m-xylene yield was 6%. The desorbent-free raffinate 2 is then sent to an o-xylene distillation column. Discharge fluid flow 0.05 liters / hour at the bottom of the tower, and its weight composition is as follows:
[0098] PX: p-xylene 1.2%
[0099] MX: m-xylene 49.4%
[0100] OX: o-xylene 49.4%.
[0101] The o-xylene yield in the bottoms was 79%.
[0102] At the top of the column, the fluid withdrawn at 0.08 l / h had the following weight composition:
[0103] EB: Ethylbenzene 0.2%
[0104] PX: p-xylene 2.1%
[0105] MX: m-xylene 89....
Embodiment 3
[0110] Adopt the same operating conditions as in Example 2, but change the flow ratio of raffinate (R2) and intermediate raffinate (R1): R2 / R1. The flow is as follows:
[0111] Material: 3.24 liters / hour
[0112] Solvent: 5.52 L / h desorbent (99.06% p-diethylbenzene and 0.94% other C 10 Aromatics)
[0113] Extraction: 3.27 liters / hour
[0114] Intermediate raffinate (R1): 3.45 liters / hour
[0115] Raffinate (R2): 2.04 liters / hour
[0116] Circulation flow (in segment 1): 16.4 l / h
[0117] The R2 / R1 ratio is 0.59.
[0118] The switching time (period) of the valve is 70.8 seconds.
[0119] After distillation of p-diethylbenzene, the resulting extract was discharged continuously to a stream of 0.71 liters / hour and a purity of 99.7% p-xylene.
[0120] 2.04 liters / hour raffinate R Distillation, obtain fluid flow rate 0.39 liters / hour, its weight composition is as follows:
[0121] EB: Ethylbenzene 0.07%
[0122] PX: p-xylene 0.98%
[0123] MX: m-xylene 69.8%
[0124] OX:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com