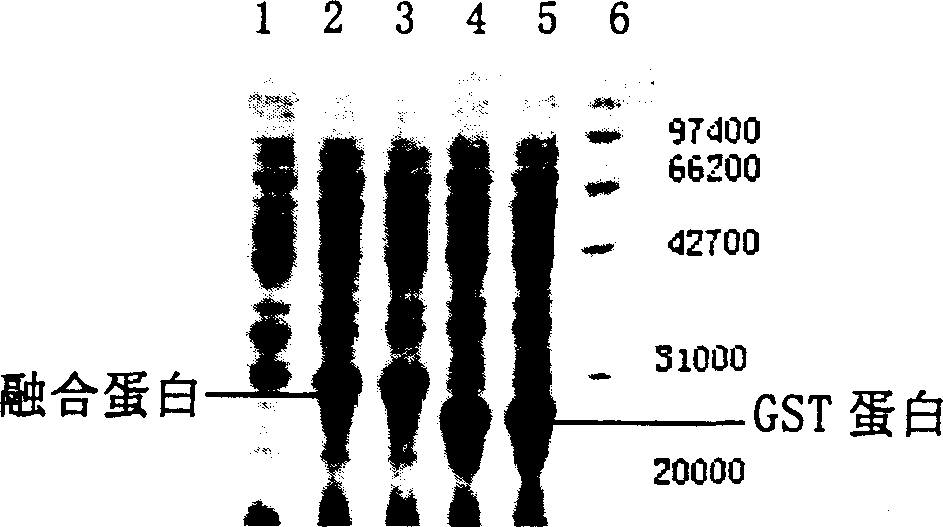Production of reorganized human parathyroid hormone 1-34 peptide
A parathyroid hormone and precursor peptide technology, applied in the field of DNA recombination, can solve the problem that the production process of PTH (1-34) peptide is not very satisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] gene synthesis
[0074] According to the amino acid sequence of the Gly-Ser-Pro-hPTH34 peptide, the preferred codons of Escherichia coli were selected, and the following 6 fragments (P1-P6) were designed and synthesized on the ABI 391 nucleic acid synthesizer:
[0075] P1.5'-cc gga tcc ccg tct gtt tct gaa atc-3' (26bp) (SEQ ID NO: 5)
[0076] P2.5'-cag ctg atg cac aac ctg ggt aaa cac ctg aac tct atg gaa cgt gtt gaa tgg-3' (54bp) (SEQ ID NO: 6)
[0077] P3.5'-ctg cgt aaa aaa ctg cag gac gtt cac aac ttc taa tag c-3' (40bp) (SEQ ID NO: 7)
[0078] P4.5'-cag ctg gat ttc ag-3' (14bp) (SEQ ID NO: 8)
[0079] P5.5'-t acg cag cca ttc a-3' (14bp) (SEQ ID NO: 9)
[0080] P6.5'-cg ctc gag cta tta gaa gtt gtg aac-3' (26bp) (SEQ ID NO: 10)
[0081] P1, P2, and P3 make up the upper chain part of the gene. The 3' end of P4 and P1 and the 5' end of P2 each have 7 bases complementary. The 3' end of P5 and P2 and the 5' end of P3 each have 7 bases complementary. P6 is complementar...
Embodiment 2
[0095] Construction of engineered bacteria
[0096] Then, the target gene constructed in Example 1 was double-digested with BamHI / XholI, ligated with the pGEX-4T-3 plasmid treated in the same way, and transfected into the host strain BL21. Positive clones were screened on the resistance plate, and a single colony was selected for culture. When the cell density reaches 0.6OD 600 , add IPTG with a final concentration of 0.4mM to induce expression screening, select a strain from the positive strains expressing the target product, extract its plasmid, and use the gene fragment P 1 ,P 6 As primers, carry out PCR reaction, and PCR amplified fragments of expected length can be seen. DNA sequence analysis was also in line with expectations (see PCR results and gene sequencing results Figure 1A ). The strain is named as Escherichia coli pGEX-PT, and its expression results are shown in Figure 1B , at a molecular weight of 30,000 Daltons, an obvious fusion protein expression band ...
Embodiment 3
[0099] Preliminary experiment of thrombin digestion and exploration of conditions for PEP digestion
[0100] (1) Exploration of PEP digestion conditions
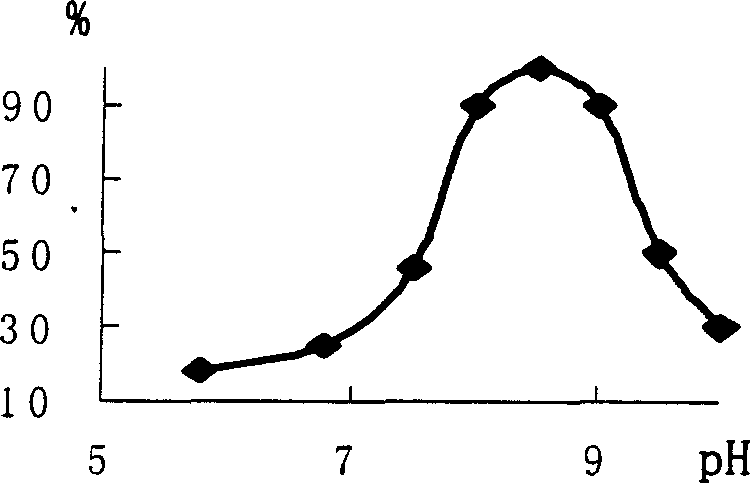
[0101] In order to determine whether the Gly-Ser-Pro-PTH (1-34) peptide in the designed fusion protein can be successfully processed by proline endonuclease, the chemically synthesized precursor 37 peptide was first used to carry out conditional experiments . The PTH(1-34) peptide precursor 37 peptide was dissolved in 20mM appropriate buffers with pH values of 5.8, 6.8, 7.5, 8.0, 8.5, 9.0, 9.5, and 10 (where pH 5.8 was phosphate buffered saline solution, and the rest of the pH values are Tris-HCl buffer), and made into 1mg / ml substrate reaction solution. PEP enzyme was added at a ratio of 1:50 (w / w). After acting on the substrate for 7 hours at 34 degrees Celsius, HPLC detects the content of 34 peptide products respectively. The experimental results show that the optimum pH for PEP to 37 peptide is 8.5 ( Figure 2A )...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com