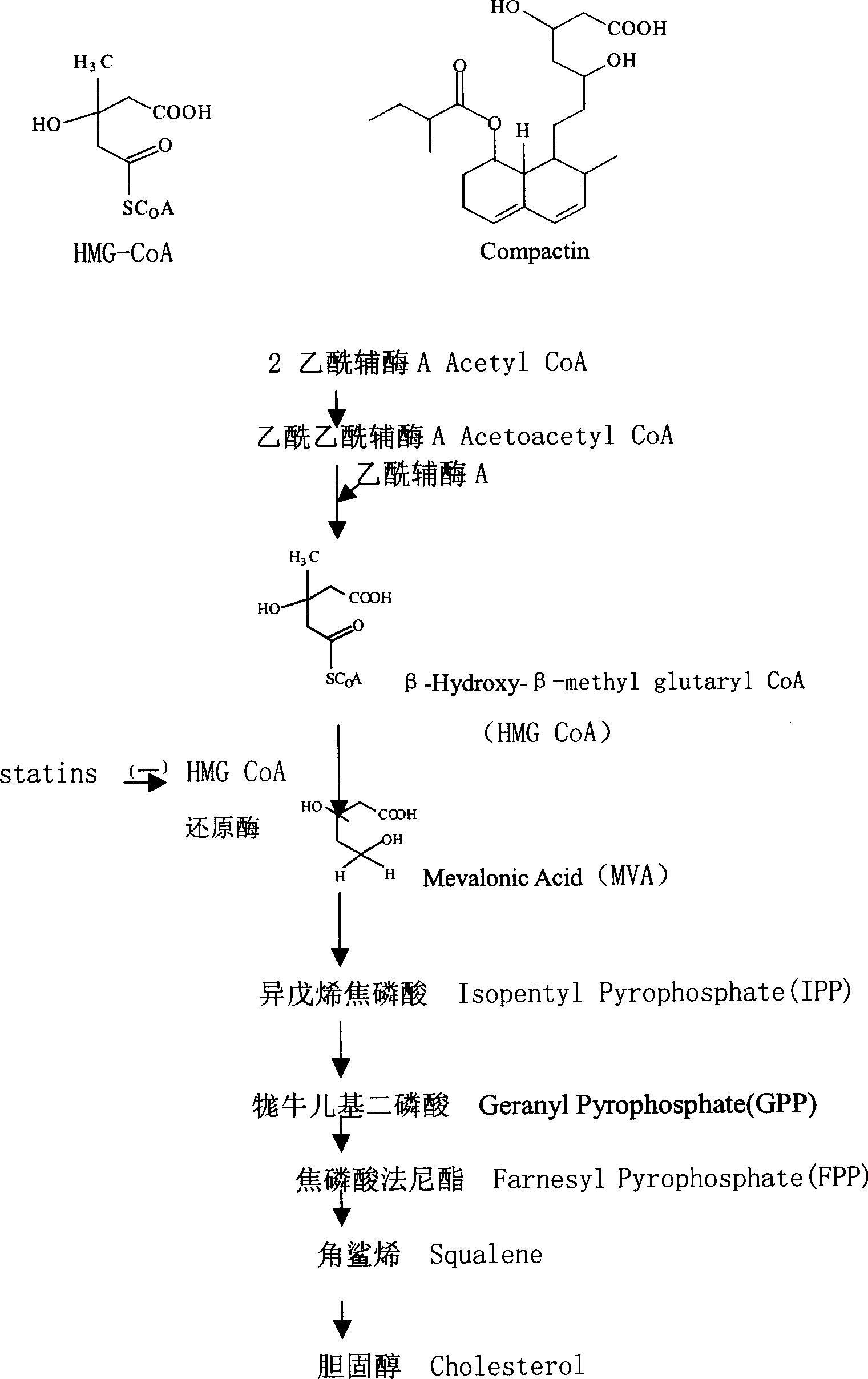
New osteoporosis-resisting medicine screening model expressed by bone formation promoting protein
A technology for anti-osteoporosis and protein expression, which is applied to the determination/testing of microorganisms, biochemical equipment and methods, etc., can solve the problems that the screening models for anti-osteoporosis drugs have not been reported, and achieve short screening cycles and high detection rates. The effect of high accuracy and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preliminary screening of 3000 strains of fungal fermentation broth
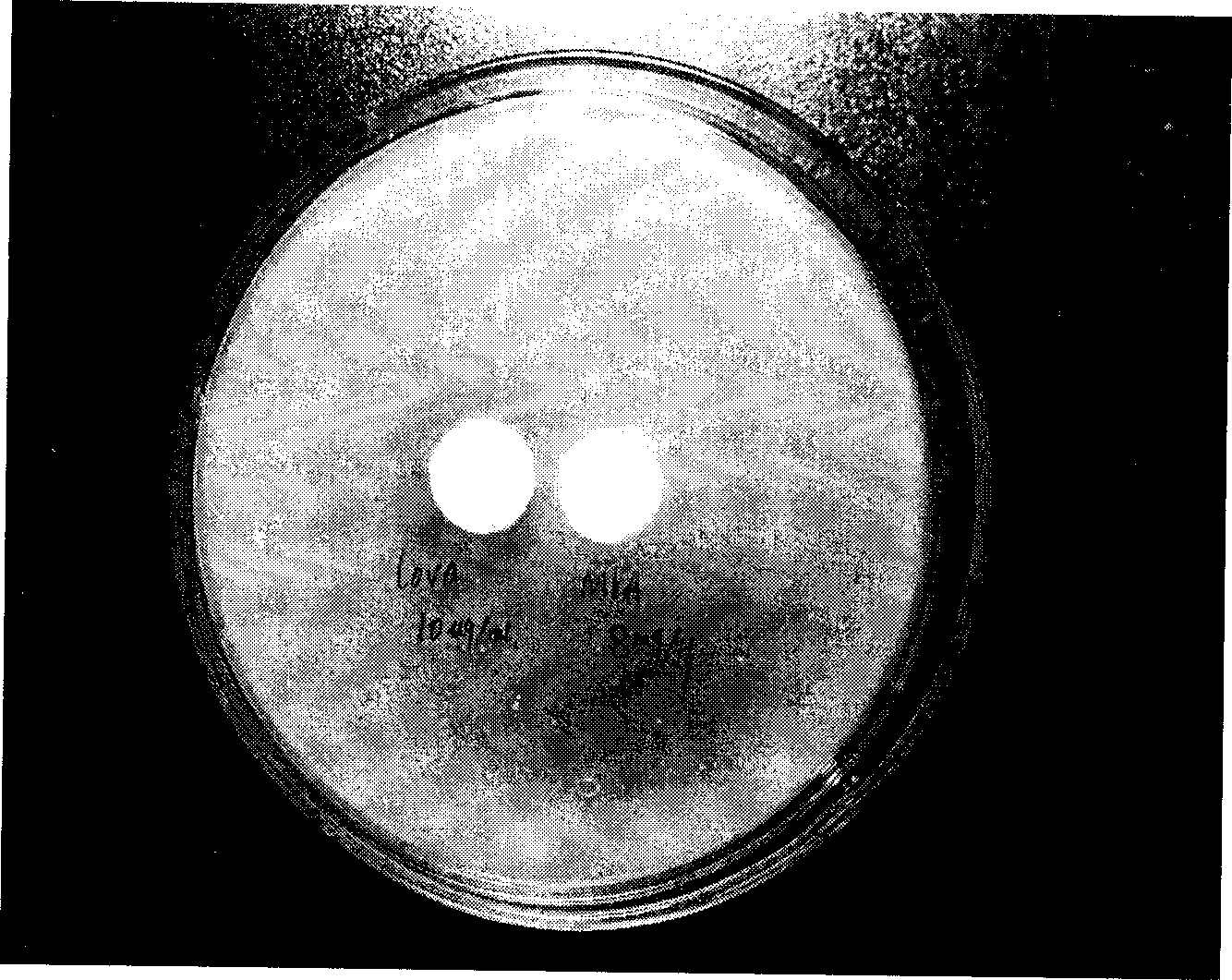
[0022] For bacterial strain fermentation, pick a small piece of cultured species from the slope and put it into a 500ml Erlenmeyer flask filled with 100ml of fermentation medium, culture it on a rotary shaker at 26°C and 120 rpm for 6 days, and take samples to measure the activity. The fermentation broth was centrifuged at 5000 rpm for 15 minutes, and the supernatant was extracted with a paper sheet, placed on a test plate inoculated with 1% SO4, incubated at 26° C. for 24 hours, and the results were observed. Among the tested samples, the diameters of the inhibition zones of the positive strains 2101, 3586, and 3879 were 27mm, 24mm, and 25mm, respectively, and the inhibitory effect was significant.
[0023] Get the above-mentioned positive bacterial strain fermentation supernatant again, place inoculated 1% SO On the assay plate, the paper sheet (8mg / sheet) containing MVA is placed next to...
Embodiment 2
[0025] Embodiment 2: to the re-screening of positive bacterial strain fermented liquid crude product
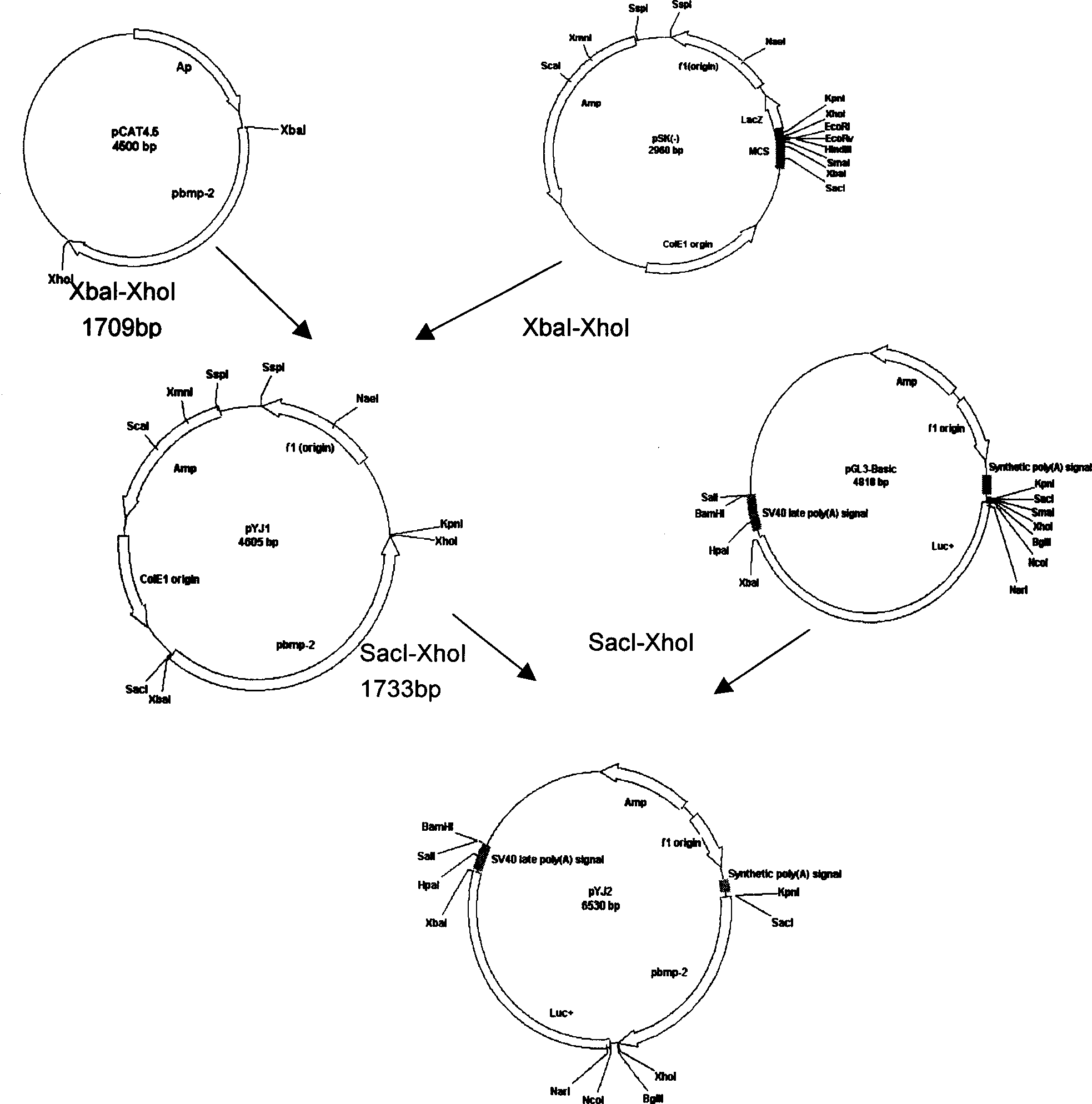
[0026] GIBCO LipofectAMINE 2000 Reagent kit was used for cell transfection: MC3T3-E1 cells were trypsinized and diluted with serum-containing DMEM medium, and quickly and evenly added to 96-well cell culture plates, counting 20,000 cells / well with a hemocytometer. Transfect after 24 hours: pYJ2 0.8 μg / well, pRL-TK 0.08 μg / well, add 25 μL of preheated serum-free DMEM medium, liposomes at a ratio of 1 μl / well, add 25 μL of serum-free DMEM medium, and transfect within 5 minutes Plasmids were mixed and left at room temperature for 20 minutes. At the same time, the medium in the plate was discarded and replaced with 100 μL serum-free DMEM medium. Add 50 μl of the mixture to each well and incubate at 37°C for 24 hours, then add the test substance (0.2 μg / ml, 2 μg / ml, 20 μg / ml control drug lovastatin and crude fermentation broth of positive strains), and continue to incubate for 24...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com