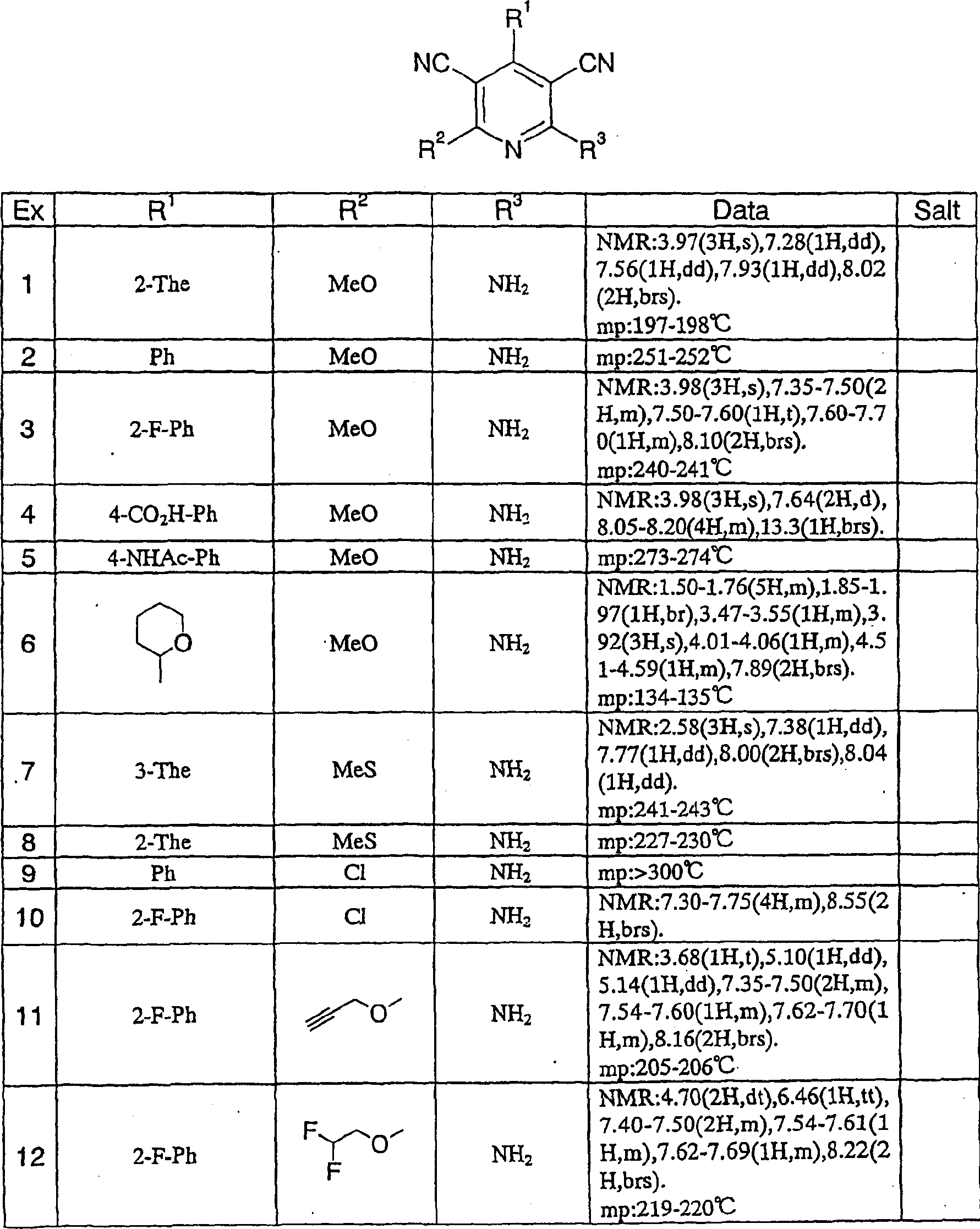
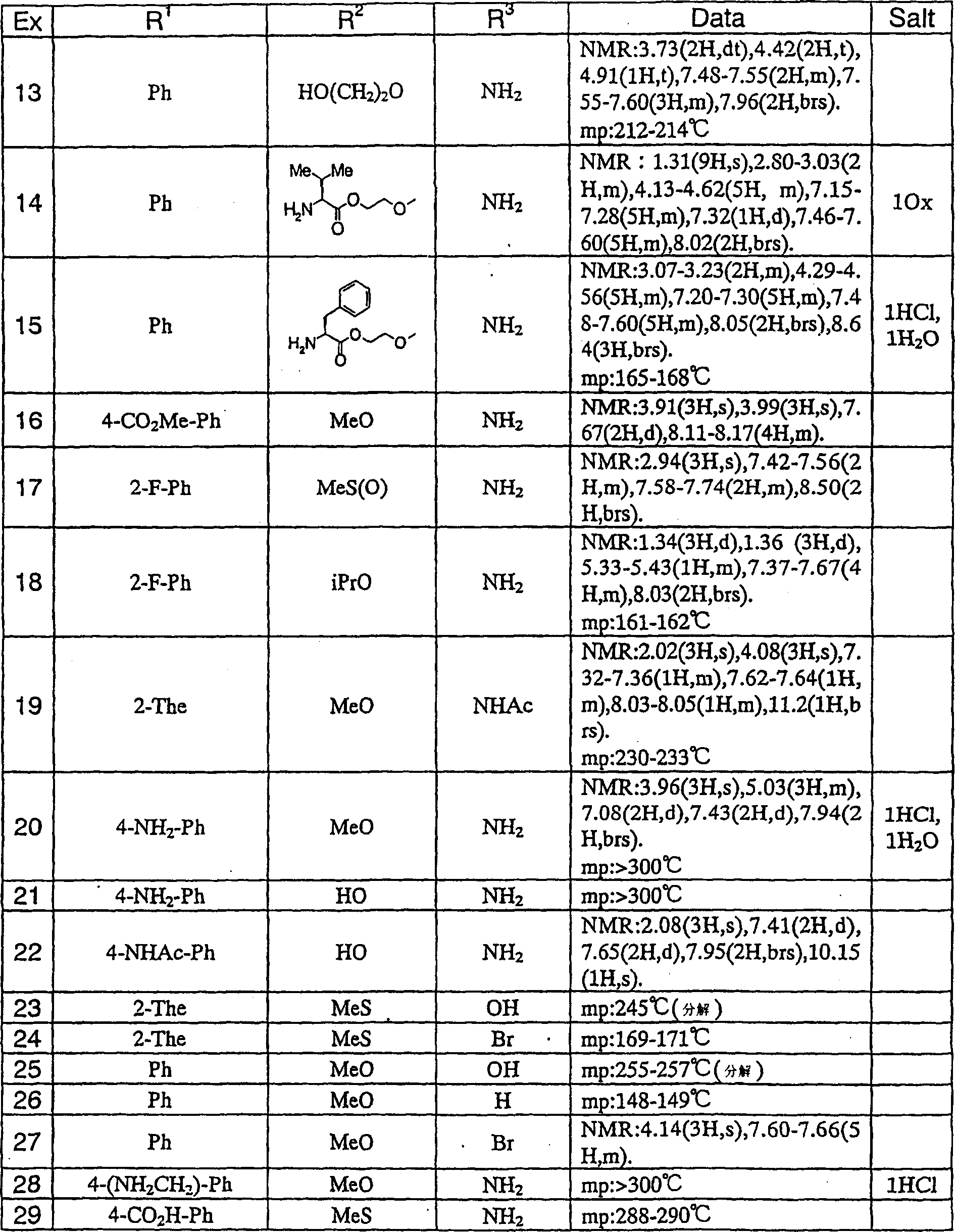
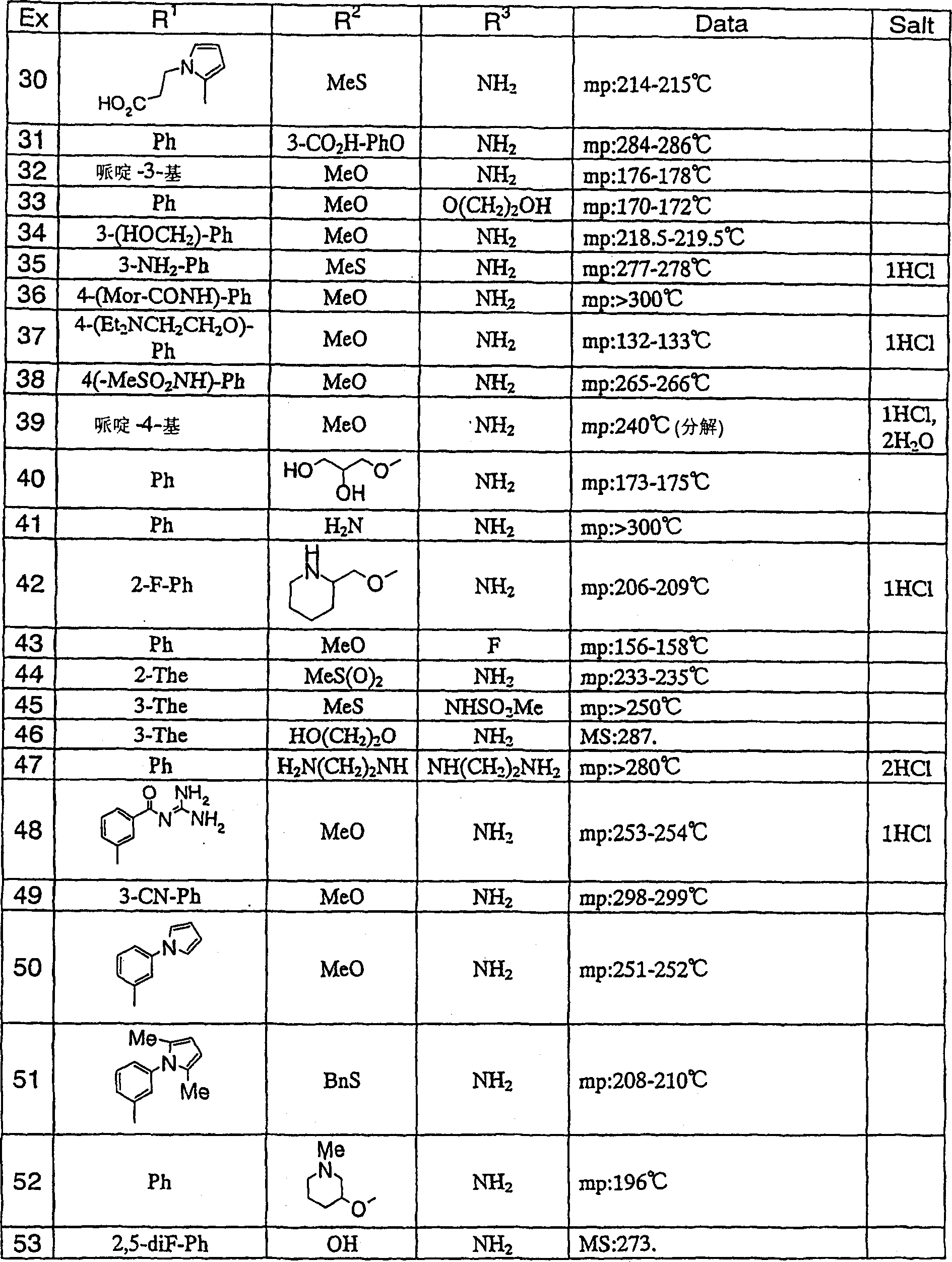
Medicine comprising dicyanopyridine derivative
A kind of technology of dicyanopyridine and alkyl, applied in the field of medicines containing dicyanopyridine derivatives, can solve the problem of no reporting relationship and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0193] To 10 ml of benzaldehyde in ethanol-water (7:3) 100 ml solution, 6.5 g of malononitrile and 0.1 g of glycine were added, and the mixture was stirred at room temperature for 6 hours. The precipitated crystals were collected by filtration, and the obtained crystals were washed with ethanol-water (7:3) and dried under reduced pressure to obtain 13.1 g of benzylmalononitrile.
[0194] As in Reference Example 1, the compounds of Reference Examples 2-7 were obtained.
reference example 8
[0196] At room temperature, add 6.0 g of sodium bicarbonate and 7.6 g of di-tert-butyl dicarbonate to a solution of 5.0 g of 4-aminomethyl benzoic acid in 40 ml of dioxane-water (1:1). The solution was 20 ml and stirred at room temperature for 4 days. The solvent was distilled off under reduced pressure, neutralized with dilute hydrochloric acid, the precipitated solid was filtered, and dried under reduced pressure to obtain 8.0 g of carboxylic acid. 0.60 g of 1,1'-carbonylbis-1H-imidazole was added to 10 ml of THF solution containing 0.85 g of carboxylic acid under ice cooling, and the mixture was stirred at 50°C for 40 minutes. In the resulting solution, add 0.43 g of N, O-dimethylhydroxylamine hydrochloride, triethylamine (Et 3 N) 0.7 mL, and stir overnight at room temperature. Water was added to the reaction solution, and extraction was performed with ethyl acetate (EtOAc). The obtained organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was distil...
reference example 9
[0198] At room temperature, in 20 ml of dioxane-water (1:1) solution of 2.0 g of 3-bromobenzylamine hydrochloride, add 1.5 g of sodium bicarbonate and 2.2 g of di-tert-butyl dicarbonate in turn. 10 ml solution of alkane was stirred at room temperature for 1 day. Water was added to the reaction solution and extracted with ethyl acetate. After the organic layer was dried with anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain 3.0 g of bromide. To a 30 ml THF solution of 3.0 g bromide, 14 ml of a 1.5M butyl lithium-hexane solution was added at -78°C, and stirred at the same temperature for 30 minutes. At -78°C, 10 ml of THF solution containing 1.7 ml of DMF was added to the resulting solution, and the temperature was raised to -15°C in 1.5 hours. An aqueous ammonium chloride solution was added to the reaction solution, and extraction was performed with ethyl acetate. The obtained organic layer was dried over anhydrous sodium sulfate, filtered, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com