Self-emulsifying agglomerant of oral polypeptide medicine
A polypeptide and self-emulsification technology, which is applied in the fields of pharmaceutical formulation, emulsion delivery, peptide/protein composition, etc., can solve the problems of bioavailability not exceeding 5%, unsatisfactory results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
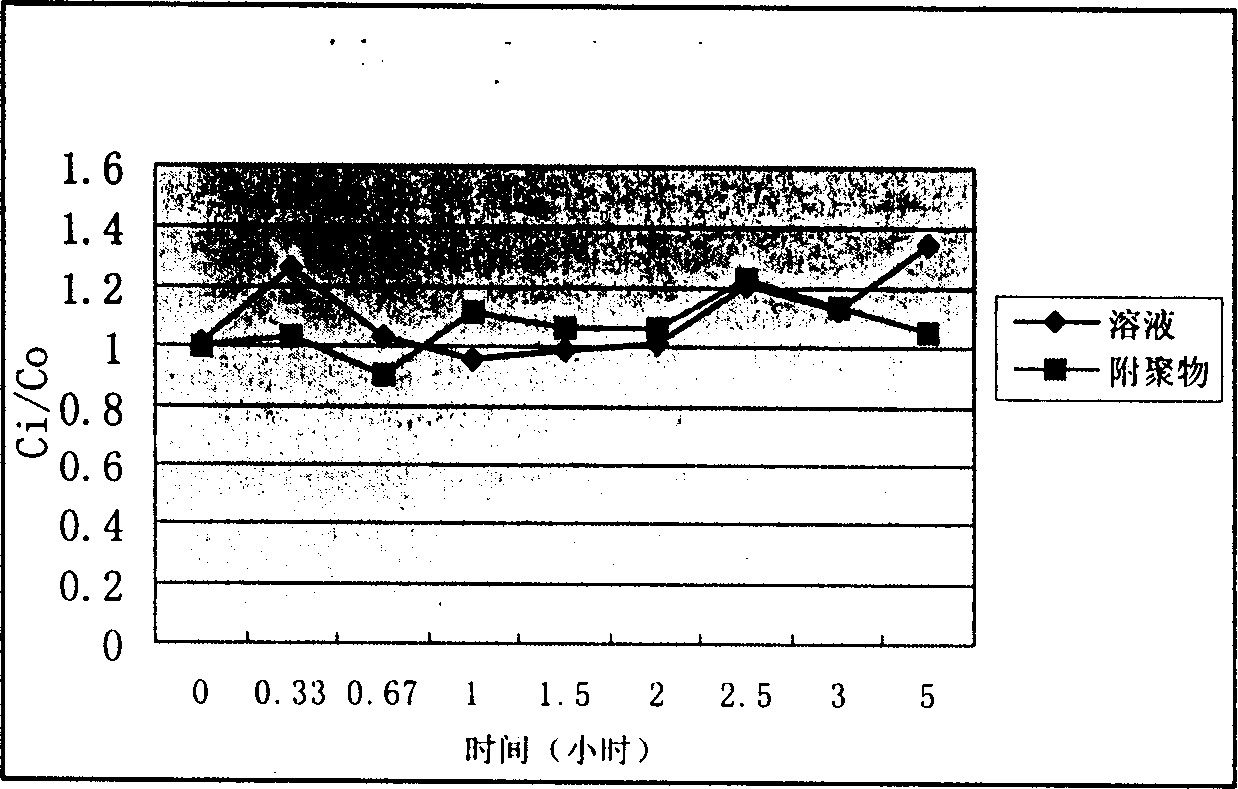
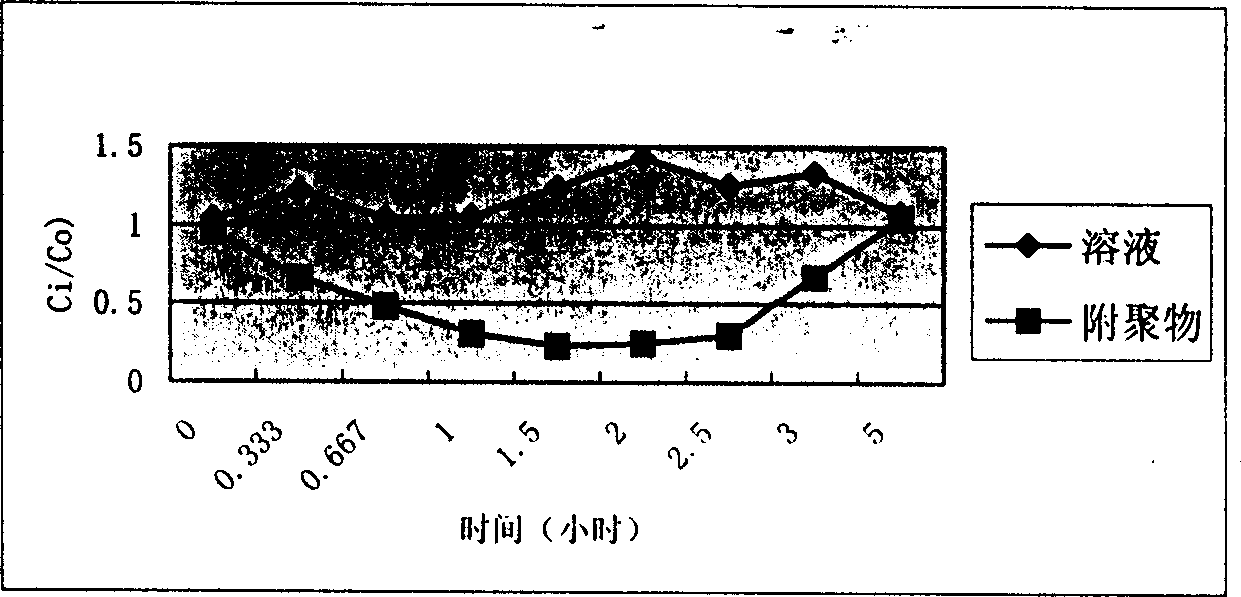
Image
Examples
Embodiment 1
[0040] Insulin (Insulin, INS) 1g, polysorbate 1g, polyoxyethylene hydrogenated castor oil 2.5g, caprylic / capric glyceride 2g, ethanol 0.5g, chitosan 0.5g, water 15ml, appropriate amount of pH regulator, dissolved After mixing, freeze-dry for 20 hours to obtain the oral self-emulsifying polypeptide drug agglomerates of the present invention.
Embodiment 2
[0042] Dissolve and mix 0.1g of parathyroid hormone, 1g of polysorbate, 2.5g of polyoxyethylene hydrogenated castor oil, 2g of peanut oil, 0.5g of ethanol, 2.5g of chitosan, and 30ml of water, and freeze-dry for 30 hours. The oral self-emulsifying polypeptide drug agglomerates of the present invention are obtained.
Embodiment 3
[0044] After dissolving 2g of INS, 0.5g of chitosan, and 10ml of 0.1N hydrochloric acid, add 2.5g of polyoxyethylene hydrogenated castor oil, 1g of peanut oil, and 50ml of water, after dissolving and mixing, directly spray-dry to obtain the oral self-administered drug of the present invention. Emulsifying peptide drug agglomerates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com