Method for quantitatively analyzing kojic dipalmitate and use thereof
A technology for quantitative analysis of kojic acid dipalmitate, applied in the field of high performance liquid chromatography analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The determination of embodiment 1 HPLC analysis condition
[0027] This embodiment adopts Waters high-performance liquid chromatography system: Alliance 2690 pump, YWG C18 250×4.6mm 10 μ m column, 2487 ultraviolet / visible light detector, Millennium 32 software system; reagents: methanol and tetrahydrofuran are dedicated to HPLC, water is distilled— Deionized water.
[0028] (1) Selection of mobile phase
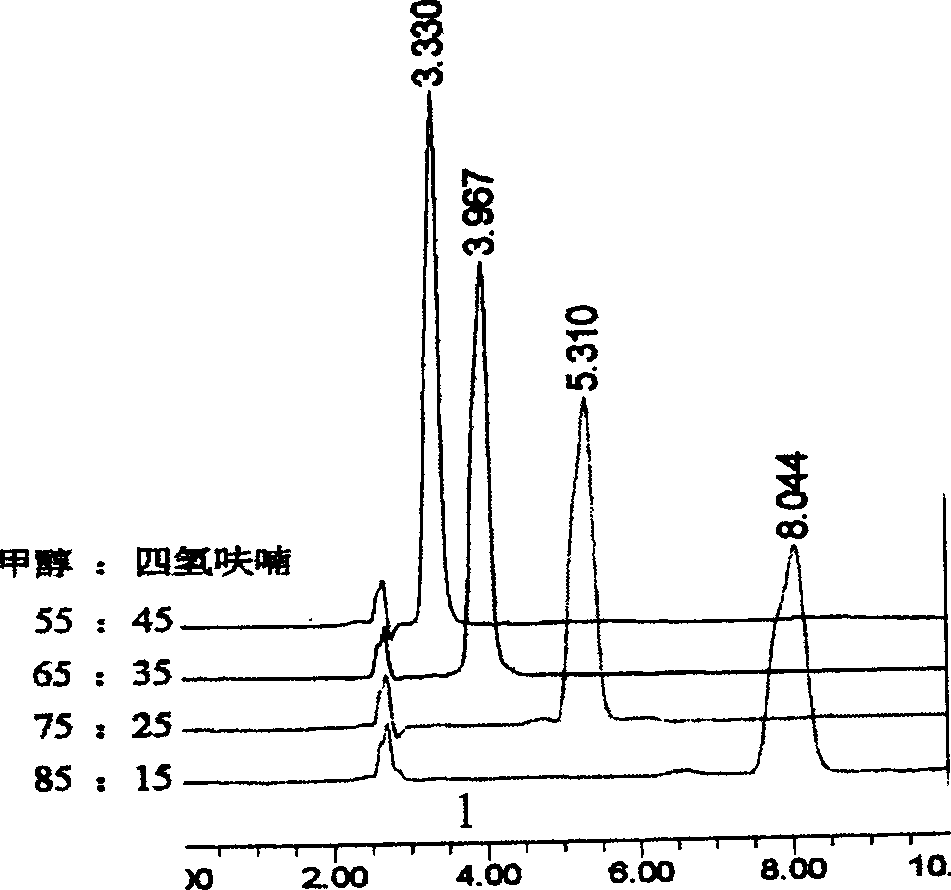
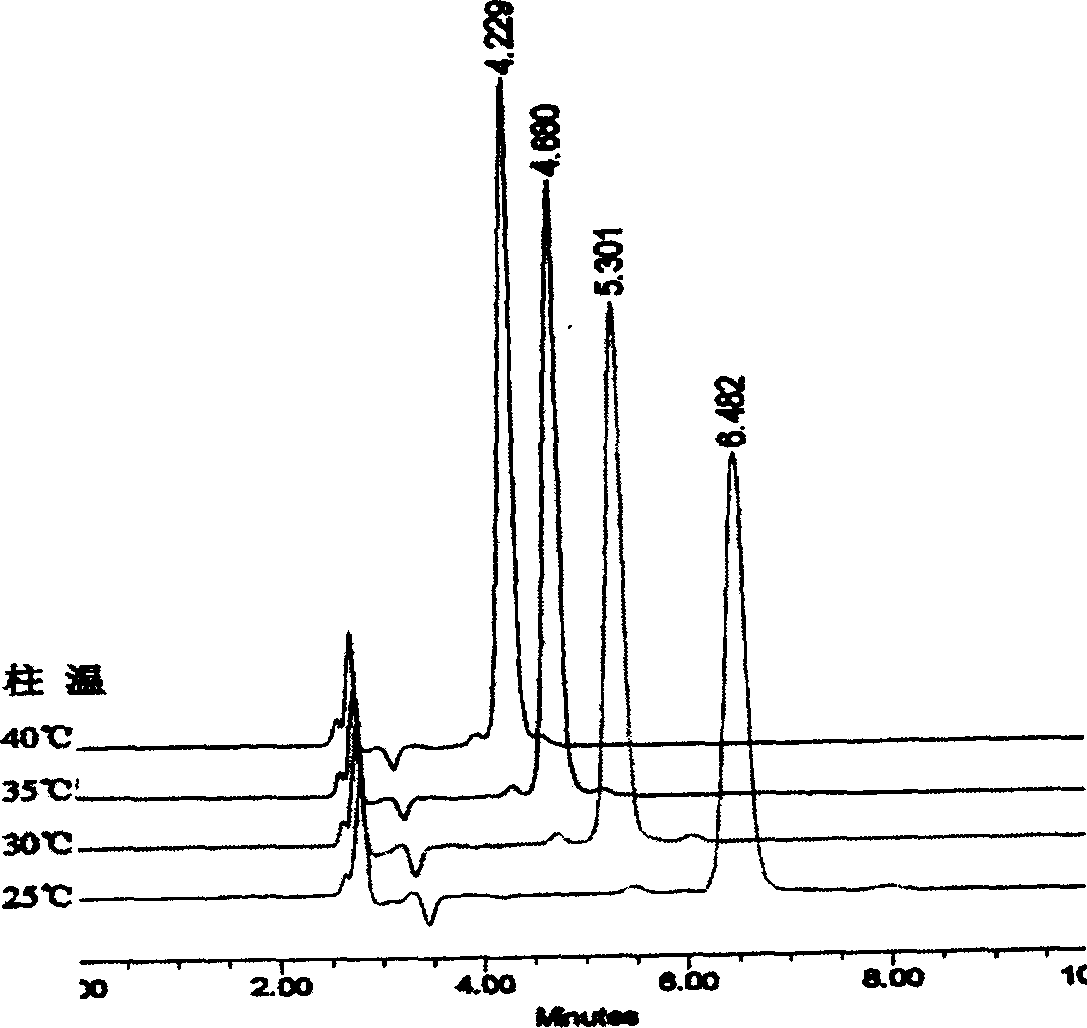
[0029] Using different ratios of tetrahydrofuran / methanol as mobile phase to make kojic acid dipalmitate chromatographic analysis, obtain a group of spectrograms ( figure 1 ). It can be seen that when the ratio of tetrahydrofuran / methanol is from 45 / 55 to 15 / 85, better separation can be achieved, while the retention time of the chromatographic peak gradually moves backward with the increase of the ratio of methanol, and the degree of separation from the impurity peak also gradually increases. The separation effect is best when the ratio of THF / methanol is 25 / 75. If...
Embodiment 2
[0038] Example 2 Quantitative correlation experiment
[0039] A group of kojic acid dipalmitate standard series samples with different contents are used for chromatographic analysis respectively according to the above analysis conditions, and the obtained chromatographic peak area and content are used for linear regression analysis, and the obtained regression curve has a good linear relationship ( Figure 4 ), correlation coefficient r=0.9995.
Embodiment 3
[0040] Embodiment 3 recovery rate and repeatability experiment
[0041] Add kojic acid dipalmitate to two skin creams that do not contain kojic acid dipalmitate, so that the content reference values are 10.0 and 100 μg / g, respectively, and after mixing thoroughly, perform parallel measurements 10 times according to this method. The results are shown in the table 1.
[0042] Table 1. Method recovery rate and repeatability test results
[0043] Determination Standard Average Average Standard Relative
[0044] Sample number Number of times Addition amount Recovery amount Recovery rate Deviation Standard deviation
[0045]Times μg / g μg / g % SD RSD%
[0046] 1 10 10 9.89 98.9 1.8 1.8
[0047] 2 10 100 99.6 99.6 1.31 1.3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com