Ligand of catalyzer for olefinic polymerization and transition metal complex
A technology for olefin polymerization and transition metal, applied in the application field of olefin polymerization catalyst in olefin polymerization, can solve the problems of affecting polymer processing performance, restricting polymer application field, narrow molecular weight distribution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
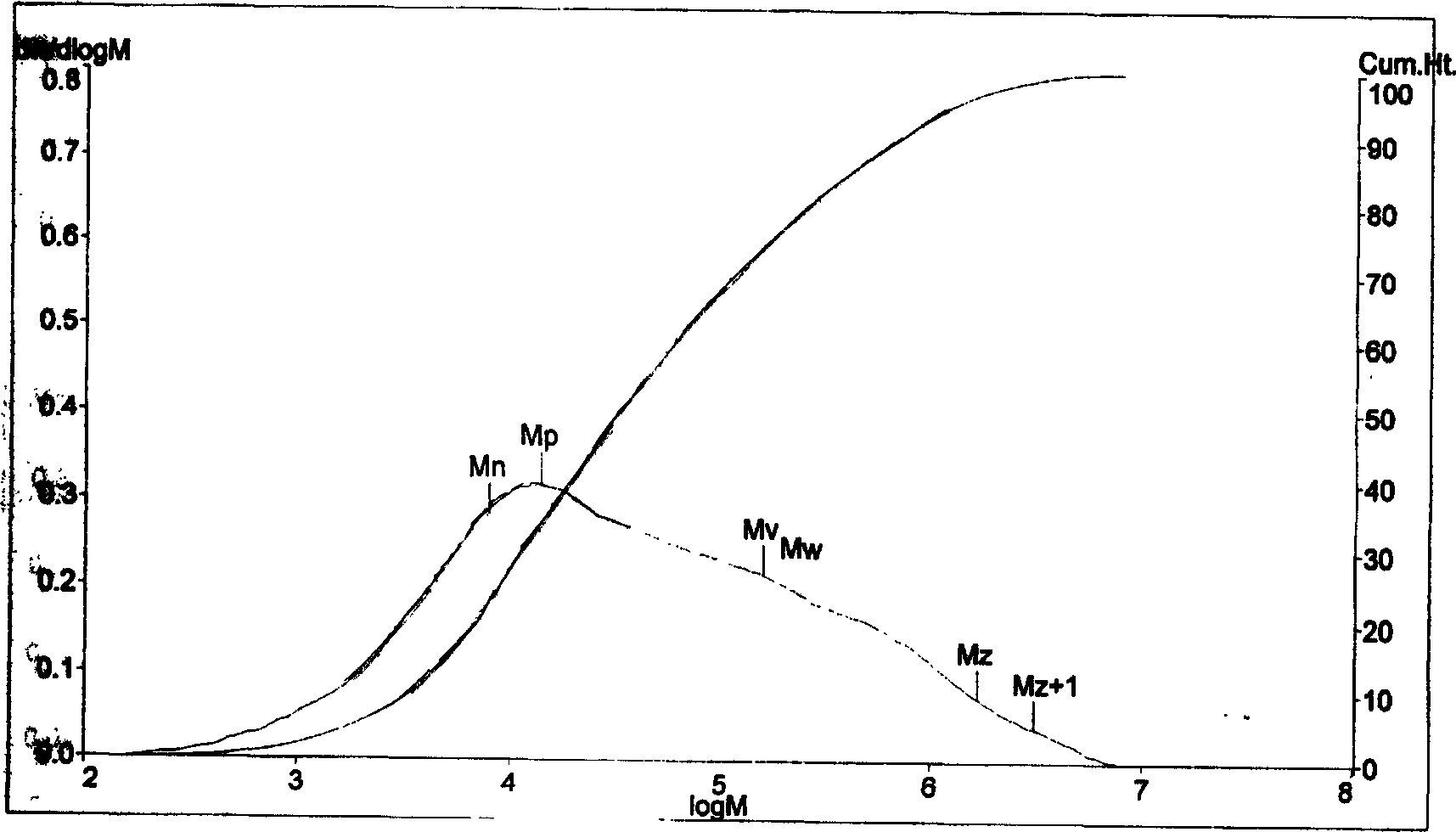
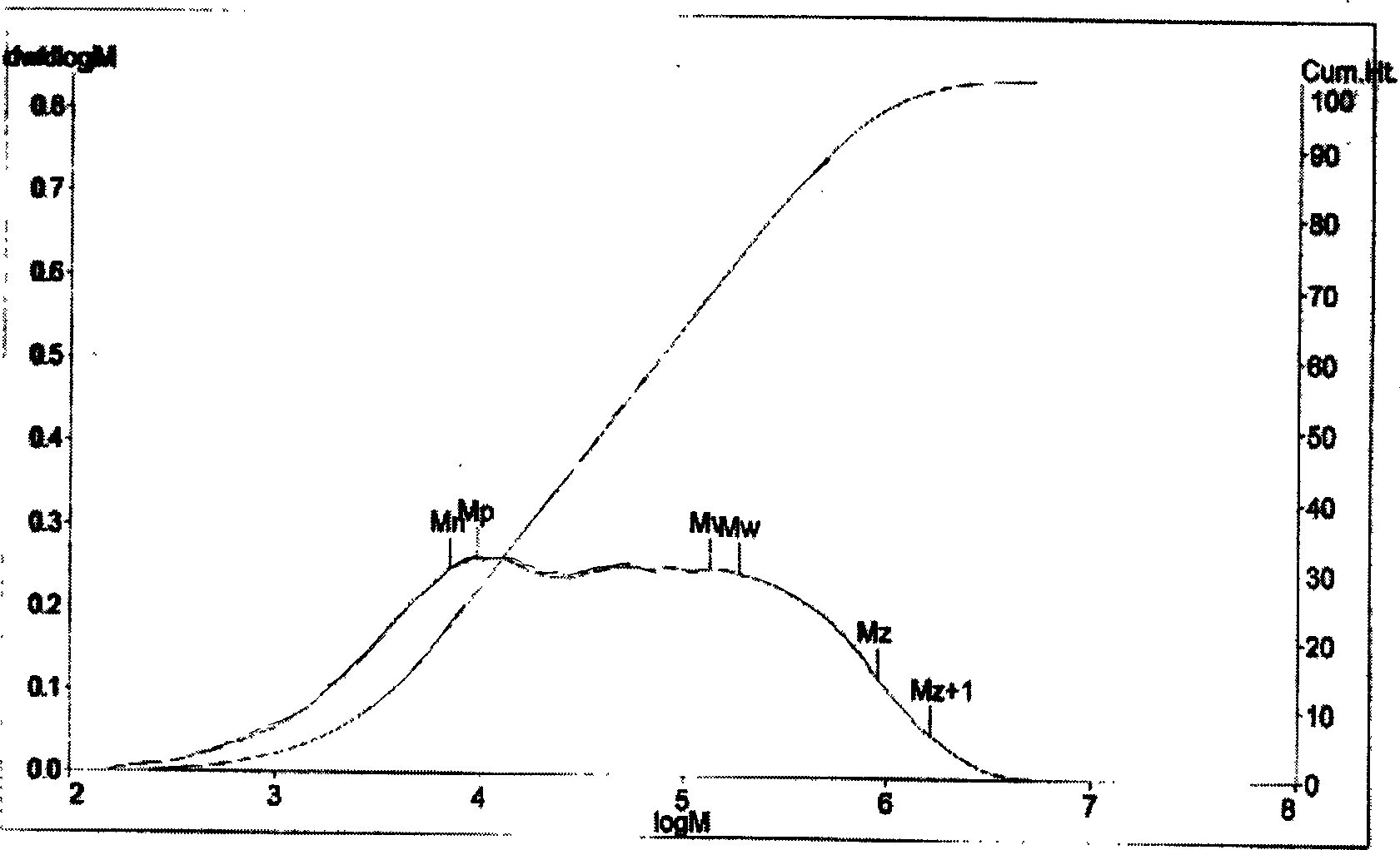
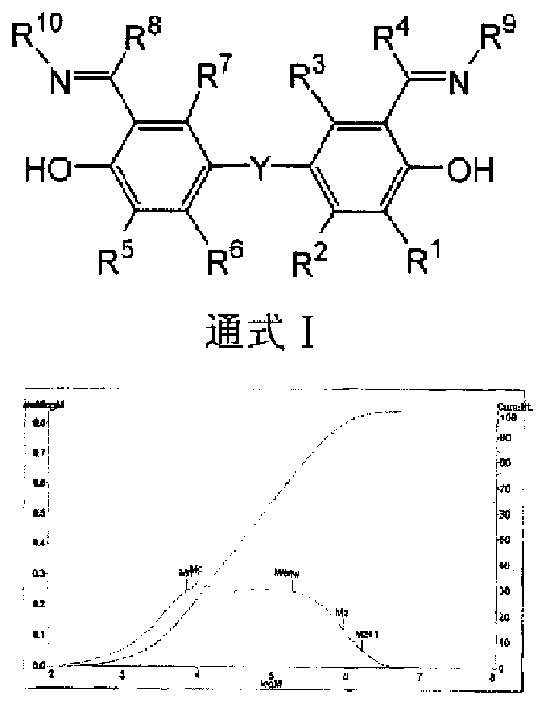
Image
Examples
Embodiment 1
[0056] Synthesis of Ligand Compound L1
[0057] 1.4, the synthesis of 4'-isopropylidene-bis(2-tert-butyl-phenol)
[0058] In a 500ml three-neck flask, add 138ml (0.6mol) of o-tert-butylphenol and 21.9ml (0.2mol) of acetone, stir, add 0.9ml of dodecanethiol, pass in hydrogen chloride gas, and react at room temperature for two days to obtain orange Viscous liquid, add 150ml of anhydrous ether, make it completely dissolved, then add 16.2g of NaHCO 3 Aqueous solution, the solution is light pink, then add an appropriate amount of distilled water to wash, collect the organic phase, add Na 2 SO 4 dry. Filter out Na 2 SO 4 , the filtrate was distilled under reduced pressure, the residue was added an appropriate amount of heptane, stirred to cool down, a large amount of white precipitate was precipitated, collected and dried to obtain white 4,4'-isopropylidene-bis(2-tert-butyl-phenol) solid powder.
[0059] Synthesis of 2.5,5'-isopropylidene-bis(3-tert-butyl-2-hydroxybenzaldehyd...
Embodiment 2
[0077] Synthetic Ligand L2
[0078] Under a nitrogen atmosphere, in a 250ml there-necked flask, add 2.0g (5.05mmol) of 5,5'-isopropylidene-bis(3-tert-butyl-2-hydroxybenzaldehyde) synthesized by the method in Example 1, and use 60ml of methanol was dissolved, then 1.39ml (12.12mmol) of cyclohexylamine and 0.6ml of formic acid were added, and the reaction was stirred at room temperature for 24 hours. The precipitate was filtered off and dried in vacuo to obtain 0.7 g (1.25 mmol, 24.8% yield) of Ligand L2 as a yellow powder.
[0079] Its structural formula is as follows:
[0080] Ligand L2CI-MS: 558 M +
Embodiment 3
[0082] synthetic ligand L3
[0083] Under a nitrogen atmosphere, in a 250ml there-necked flask, add 1.5g (3.79mmol) of 5,5'-isopropylidene-bis(3-tert-butyl-2-hydroxybenzaldehyde) synthesized by the method in Example 1, and use 60ml of methanol was dissolved, then 1.2ml (9.10mmol) of n-hexylamine and 0.6ml of formic acid were added, and the reaction was stirred at room temperature for 24 hours. The precipitate was filtered off and dried in vacuo to give ligand L3 as a yellow viscous liquid.
[0084] Its structural formula is as follows:
[0085] Ligand L3
[0086] EI-mass spectrum: 562 M +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com