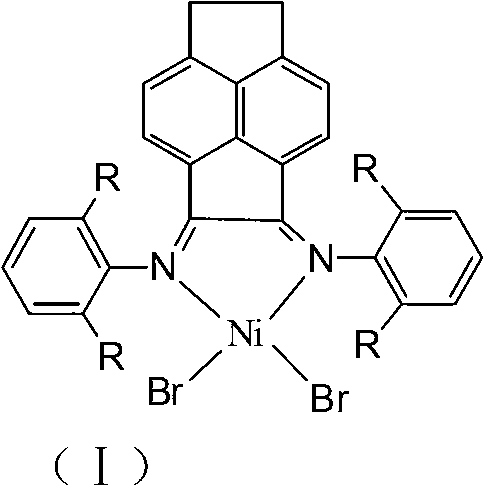
Ethylidene acenaphthene (alpha-diimine) nickel olefin catalyst, and preparation method and application thereof
An olefin catalyst, the technology of ethylene acenaphthene, which is applied in the field of ethylene acenaphthene (α-diimine) nickel olefin catalyst and its preparation, can solve the problem of high aluminum-nickel ratio, which is not conducive to industrialization and commercialization, and catalyst ligands Complex process and other issues, to achieve the effect of high activity, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of the described ethylene acenaphthylene (α-diimine) nickel olefin catalyst is characterized in that: its steps are as follows:
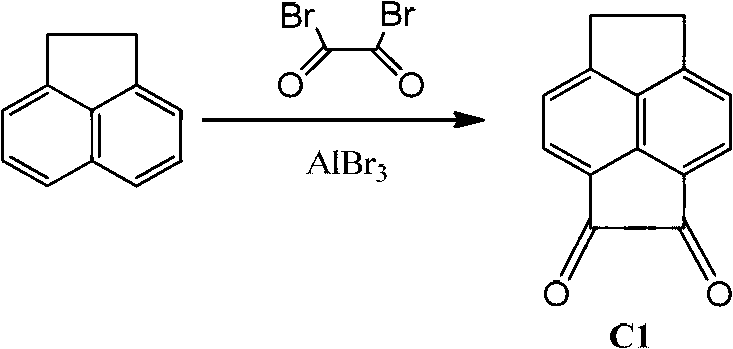
[0026] 1) Compound C1 is obtained by diacylation of acenaphthoquinone: using acenaphthoquinone as raw material and carbon disulfide as solvent. Adding anhydrous aluminum bromide, under the condition of oxalyl bromide as the oxidizing agent, the yellow solid acenaphthyldione (C1) was obtained.
[0027]
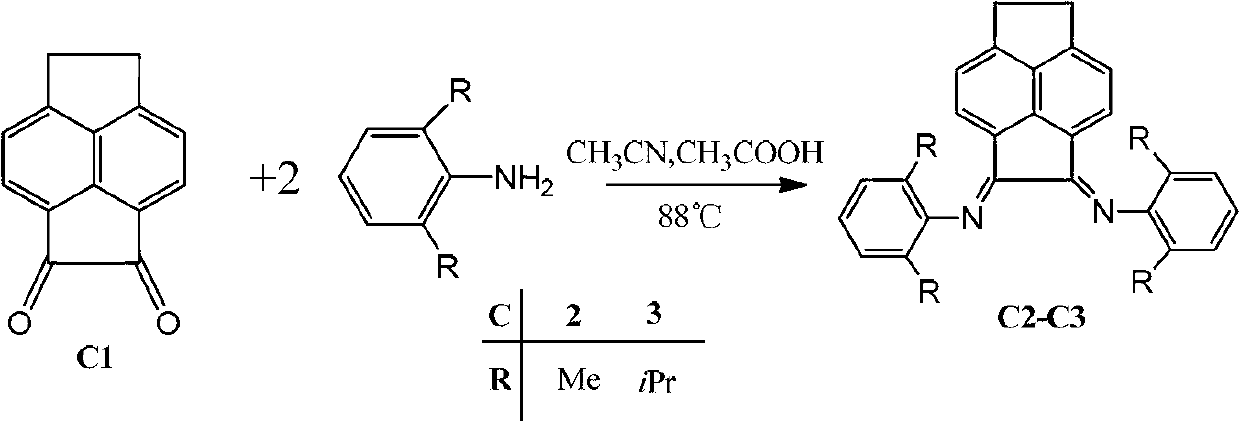
[0028] 2) Compound C1 undergoes ketoamine condensation reaction with symmetrical aniline to obtain α-diimine ligand C2 or C3. The acenaphthedione (C1) raw material obtained in step 1) is reacted, acetonitrile is used as a solvent, acetic acid is used as a catalyst, and α-diimine ligand (C2 or C3) is obtained through ketone-amine condensation reaction.
[0029]
[0030] 3) Under anhydrous and oxygen-free conditions, the α-diimine ligand C2 or C3 and (DME)NiBr 2 Complexation, the olefin polymerization catalyst (C4,...
Embodiment 1
[0038] Add 0.305g (1.47mmol) of C1 into a 100mL three-neck flask, add 30mL of acetonitrile, reflux at 88°C for one hour, add 7mL of acetic acid, and reflux for another four hours. Then, 0.68 mL (3.23 mmol) of 2,6-diisopropylaniline was added and reacted for 24.5 hours. After the reaction, the solvent was drained, and the crude product was separated by column chromatography (petroleum ether / dichloromethane: 1 / 1, 0.75% triethylamine, silica gel) to obtain 0.301 g of the product with a yield of 38.9%. 1 H-NMR (300MHz, CDCl 3 ,δin ppm):7.50(s,6H,Ar-H),6.45-7.15(dd,4H,Py-H),2.98-3.14(sept,4H,CH(CH 3 ) 2 ),0.86-1.36(dd,24H,CH(CH 3 ) 2 ).
[0039] Elem.Anal.Calcd.For C 38 h 42 N 2 :C,86.65%;H,8.04%;N,5.32%.Found:C,86.09%;H,8.04%;N,5.31%.
[0040] ESI-MS: m / z 527.10 ([M+H] + .
Embodiment 2
[0042] 0.290g (1.40mmol) of C1 was added to a 100mL three-neck flask, 30mL of acetonitrile was added, refluxed at 88°C for one hour, 7mL of acetic acid was added, and refluxed for another four hours. Then, 0.65 mL (3.07 mmol) of 2,6-dimethylaniline was added and reacted for 24 hours. After the reaction, the solvent was drained, and the crude product was separated by column chromatography (petroleum ether / dichloromethane: 1 / 1, 0.75% triethylamine, silica gel) to obtain 0.249 g of the product, with a yield of 42.9%.
[0043] Elem.Anal.Calcd.For C 30 h 26 N 2 :C,86.09%;H,8.04%;N,5.31%.Found:C,86.82%;H,6.36%;N,6.56%.
[0044] ESI-MS: m / z 415.3 ([M+H] + .
[0045] 2. Preparation of ethylene acenaphthylene α-diimine nickel complex
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com