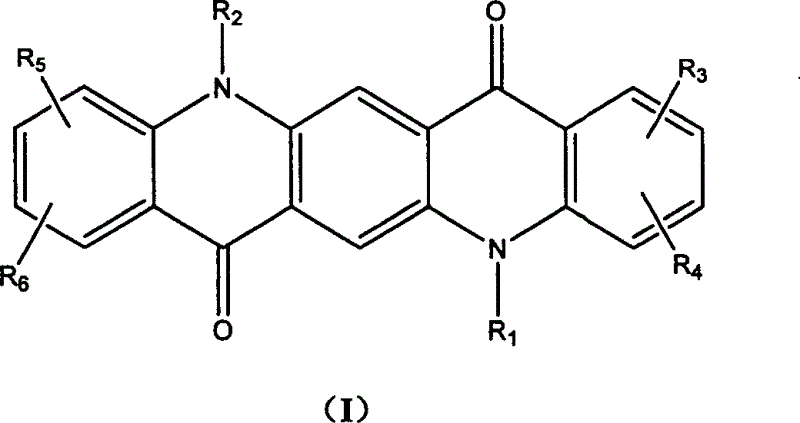
Quinacridone derivant and application in organic electroluminescent device thereof
A quinacridone and derivative technology, applied in the field of organic electroluminescent materials, can solve the problems of harsh device process requirements, fluorescence quenching, etc., and achieve the effects of good stability, low turn-on voltage and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
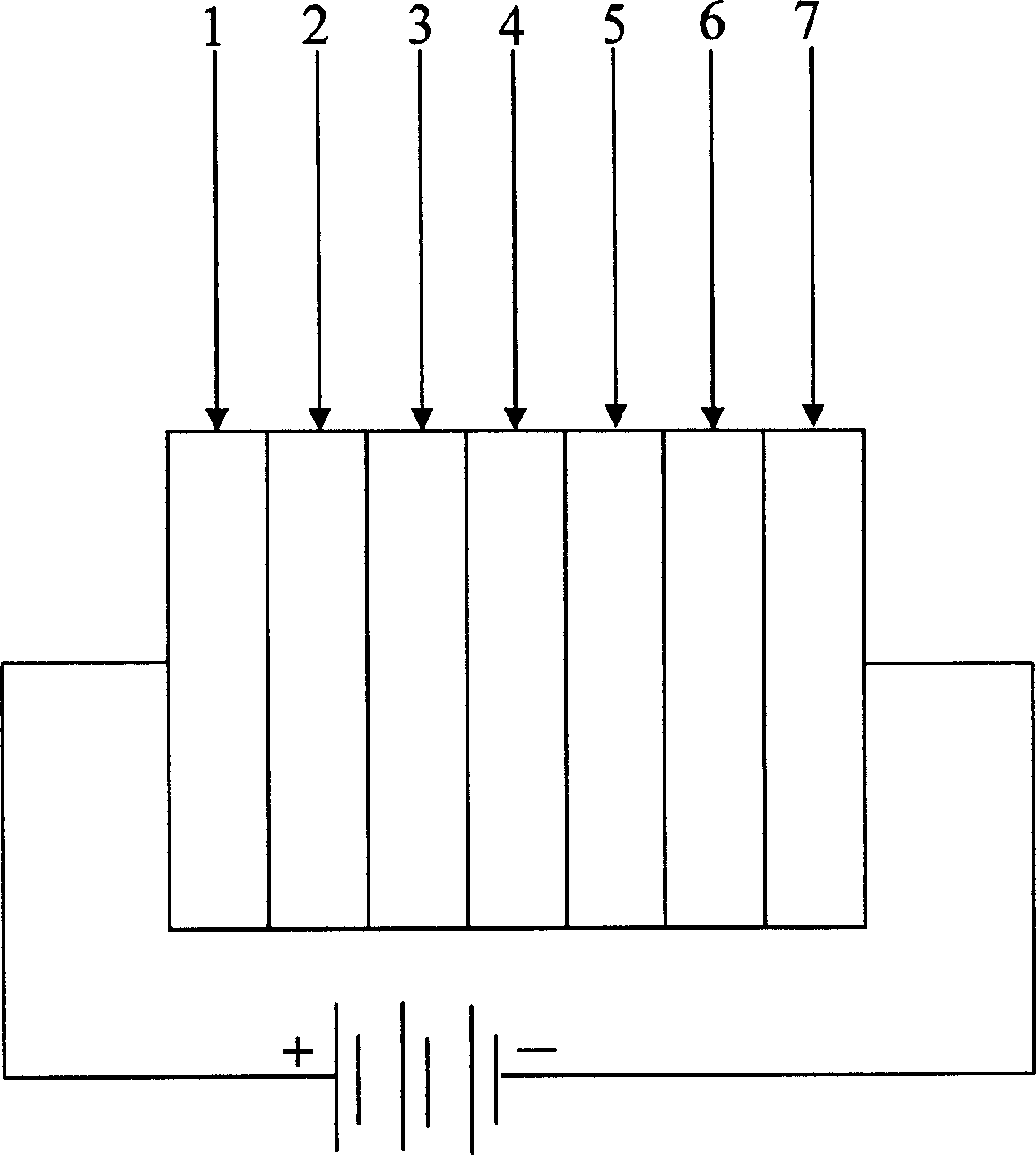
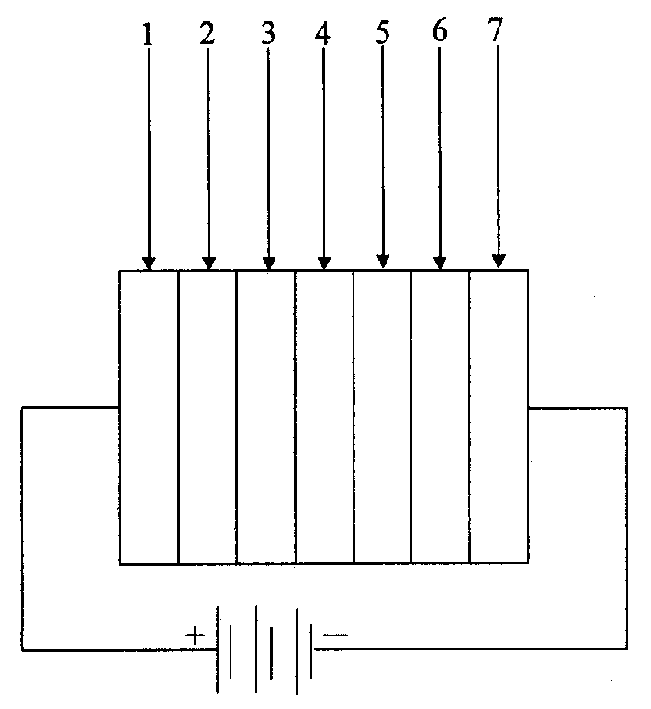
Image
Examples
example 1
[0024] The following examples are used to further illustrate the preparation and application of the compounds of the present invention, instead of limiting the present invention by using these examples. Example 1: Synthesis of HMQA
[0025] Using 120ml of absolute ethanol as solvent, add 10.0g of compound 2,5-dihydroxy-1,4-cyclohexadiene-1,4-dicarboxylate, 20ml of 3,5-dimethylaniline, and hydrochloric acid 1ml, heated to reflux for 6 hours. Cool and filter, wash the precipitate with ethanol to obtain 16.3 g of pink product 2,5-bis(3,5-dimethylanilino)-1,4-cyclohexadiene-1,4-dicarboxylic acid diethyl ester, The yield was 90.3%.
[0026] 10.0 g of the compound 2,5-bis(3,5-dimethylanilino)-1,4-cyclohexadiene-1,4-dicarboxylic acid diethyl ester and 1-chloronaphthalene were heated to reflux at 260°C 2 After hours, filter and wash with chloroform, 6.5 g of 1,3,8,10-tetramethyl-6,13-dihydro-quinacridone is obtained, and the yield is 81.2%.
[0027] Add 5.0 g of compound 1,3,8,10-tetramet...
example 2
[0031] HMQA Example 2: Synthesis of TMDEQA
[0032] The synthesis of TMDEQA is the same as in Example 1. Only bromoethane is used instead of methyl iodide. The product 1,3,8,10-tetramethyl-5,12-diethylquinacridone (TMDEQA). Mass spectrum molecular ion peak: 425. Elemental analysis according to chemical formula C 28 H 28 N 2 O 2 Calculation: C: 79.2%; H: 6.6%; N: 6.6%; experimental value: C: 79.1%; H: 6.8%; N: 6.5%.
[0033]
[0034] 5,12-Diethyl-1,3,8,10-tetramethyl-quinacridone
example 3
[0035] TMDEQA Example 3: Synthesis of TMDPQA
[0036] The synthesis of TMDPQA is the same as in Example 1. Only 1-bromopropane is used instead of methyl iodide. The product 1,3,8,10-tetramethyl-5,12-dipropylquinacridone (TMDBA). Mass spectrum molecular ion peak: 453. Elemental analysis according to chemical formula C 30 H 32 N 2 O 2 Calculation: C: 79.6%; H: 7.1%; N: 6.2%; experimental value: C: 79.3%; H: 7.3%; N: 6.0%.
[0037]
[0038] 1,3,8,10-Tetramethyl-5,12-dipropyl-quinacridone
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com