Rareearth beteropoly acid salt (blue) group anti-AIDS medicine and preparation process thereof
A heteropoly acid salt and anti-AIDS technology, which is applied in the field of rare earth heteropoly acid salt compounds to achieve the effects of low price, strong virus replication and strong acid-base stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the preparation of HPB-1
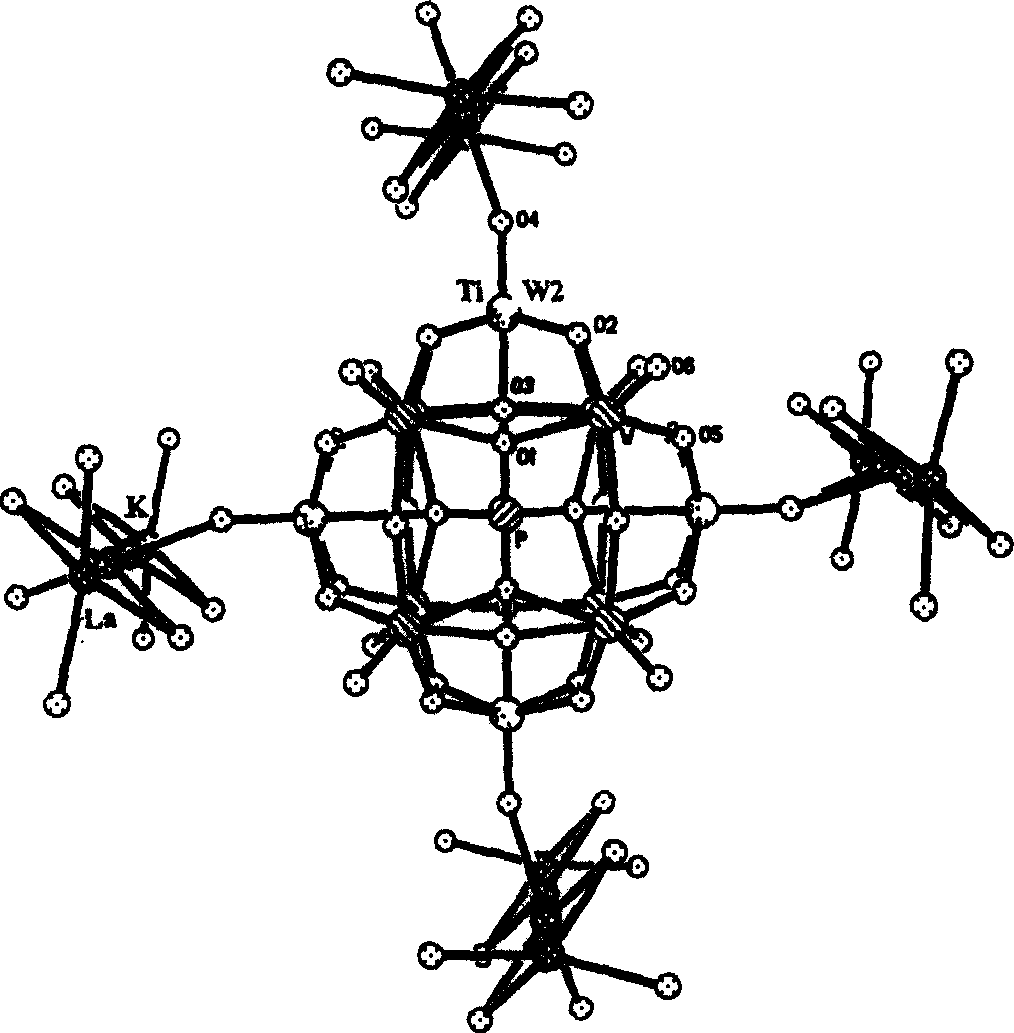
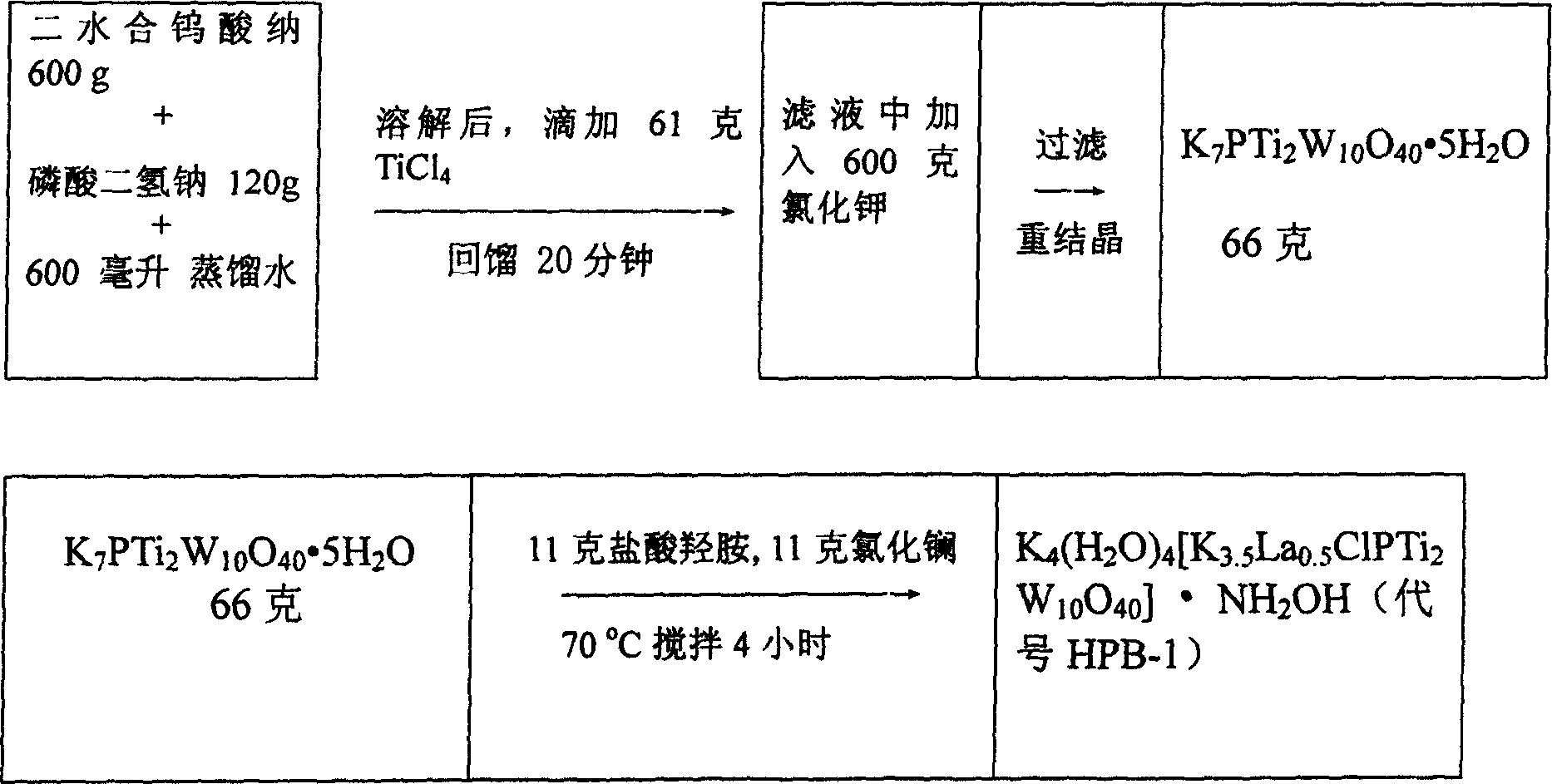
[0092] Sodium tungstate dihydrate (600 grams, 1.82mol), sodium dihydrogen phosphate (120 grams, 0.86mol) were dissolved in 2000 grams of distilled water, titanium tetrachloride (61 grams, 0.32mol) was added dropwise, back distillation 20 minutes, After filtration, 600 g of solid KCl was added to the filtrate to obtain a white precipitate. The precipitate was recrystallized with 500 g of hot water to give solid K 7 PTi 2 W 10 o 40 ·5H 2 O66g, K 7 PTi 2 W 10 o 40 ·5H 2 Dissolve O66g in 500g of water, add 11g of hydroxylamine hydrochloride and stir, then add 11g of lanthanum chloride, stir at 40-80°C for 2-8 hours, a few days later a colorless crystal K will precipitate 4 (H 2 O) 4 [K 3.5 La 0.5 (H 2 O) 8 ClPTi 2 W 10 o 40 ]·NH 2 Oh. The molecular structure diagram is attached figure 1 . The HPB-1 production flow chart is attached figure 2 .
Embodiment 2
[0093] Embodiment 2: the preparation of HPB-2
[0094] Prepare K by the method of above-mentioned embodiment 1 7 PTi 2 W 10 o 40 ·5H 2 O, K 7 PTi 2 W 10 o 40 ·5H 2 Dissolve O66 g in 500 g of water, add 9 g of hydrazine hydrate and stir, then add 12 g of neodymium chloride, and stir at 40-80°C for 2-8 hours. After a few days, purple crystals of K were precipitated 4 (H 2 O) 4 [K 3.5 Nd 0.5 (H 2 O) 8 ClPTi 2 W 10 o 40 ]·NH 2 NH 2 (HPB-2) 80g.
[0095] The preparation method of the above examples 1 and 2 can be arbitrarily enlarged or reduced according to the mass ratio of the formula.
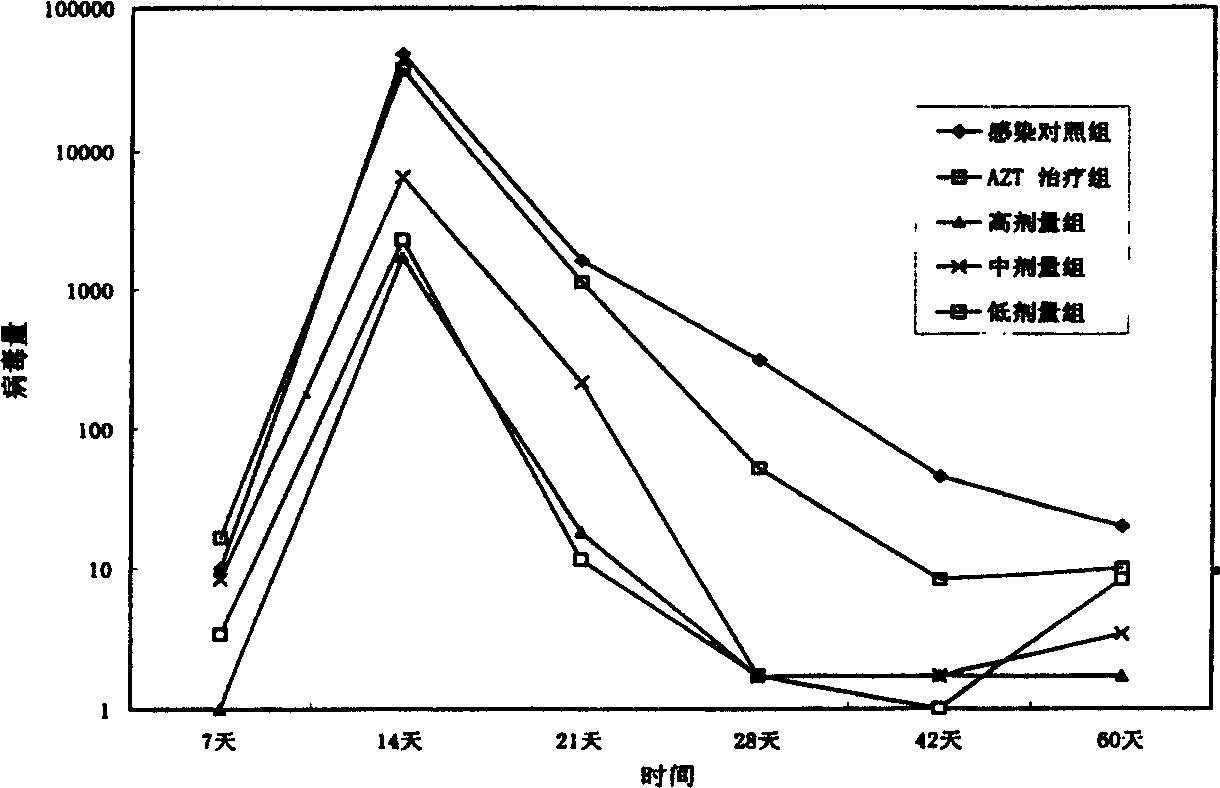
[0096] The synthesized HPB-1, HPB-2 and excipients can be made into capsules at a weight ratio of 1:3. According to the ratio of its weight to the excipient of 1:3, it is made into a compressed tablet. Add water for injection according to the conventional injection preparation method, filter, potting and sterilize to make injection.
Description of drawings
[0097] atta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com