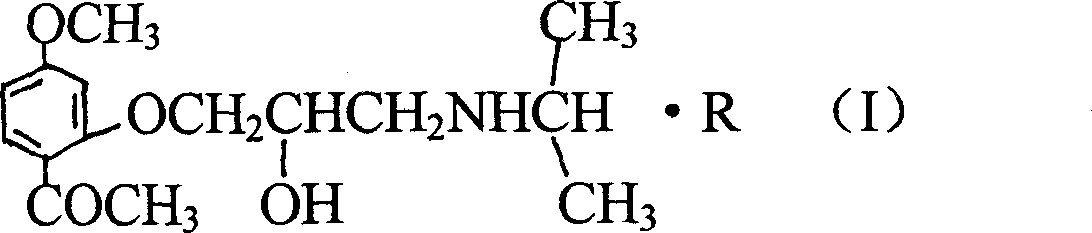
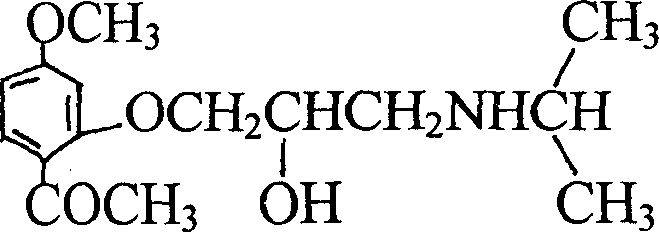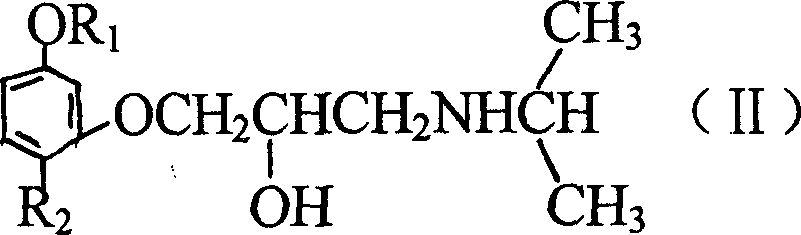Propanol amine kind ether compound synthesized using paeonol as mother body and its derivative
A technology of propanolamine ethers and ether compounds, which is applied in the field of compounds, can solve problems that have not been reported, and achieve the effects of enhanced prevention and control, less toxic and side effects, and strong pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of paeonolol (namely the preparation of the etherified compound of propanolamines with paeonol as parent)
[0040] The chemical name of Dampirol is: 1-isopropylamino-3-(2-acetyl-5-methoxy-phenoxy)-2-propanol, and its structural formula is as follows:
[0041]
[0042] Take 25 grams of paeonol and 40 milliliters of epichlorohydrin, add 7 grams of sodium hydroxide, react at 90 ° C for 3 hours, add 50 milliliters of water, separate the water layer, evaporate the water, add 60 milliliters of isopropylamine to react After 10 hours, excess isopropylamine was evaporated, 40 ml of xylene was added, and the mixture was left overnight. The precipitate was recrystallized with ethanol to obtain 21.5 g of product.
[0043] m.p.129~131℃.
[0044] IR:
[0045] 1253 (Ar-O-), 1602 (C=C), 1425(-CH 3 ),
[0046]
[0047] 2.82(1H, Sept, J=6.0Hz, 2.67-2.77 (1H, m, N-CH), 2.87-2.96 (1H, m, N-CH), 3.84 (3H, s, CH) 3 O), 4.05 (3H, br, s + m, OCH, O...
Embodiment 2
[0054] Example 2: Preparation of 1-isopropylamino-3-(2-propionyl-5-methoxy-phenoxy)-2-propanol
[0055] Take 25 g of 2-hydroxy-4-methoxy-propiophenone and 40 ml of epichlorohydrin, add 7 g of sodium hydroxide, react at a temperature of 100 ° C for 3 hours, add 50 ml of water, separate the water layer, evaporate Remove water, add 60 ml of isopropylamine to react for 10 hours, evaporate excess isopropylamine, then add 40 ml of xylene, leave overnight, and recrystallize the precipitate with ethanol to obtain 20.1 g of product.
[0056] m.p.132~140℃.
Embodiment 3
[0057] Example 3: Preparation of 1-isopropylamino-3-(2-acetyl-5-ethoxy-phenoxy)-2-propanol
[0058]Take 25 g of 2-hydroxy-4-ethoxy-acetophenone and 40 ml of epichlorohydrin, add 7 g of sodium hydroxide, react at a temperature of 110 ° C for 5 hours, add 50 ml of water, and remove the water layer, The water was evaporated, 60 ml of isopropylamine was added to react for 11 hours, the excess isopropylamine was evaporated, 40 ml of xylene was added, and the mixture was left overnight. The precipitate was recrystallized with ethanol to obtain 19.6 g of product.
[0059] m.p.143~148℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com