Fluorene-naphthalene imide derivative compound and its application
A naphthalene diimide compound technology, applied in the field of fluorene-naphthalene diimide derivative compounds, to achieve the effects of good solubility, good electron transport, and excellent electron injection and transport capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
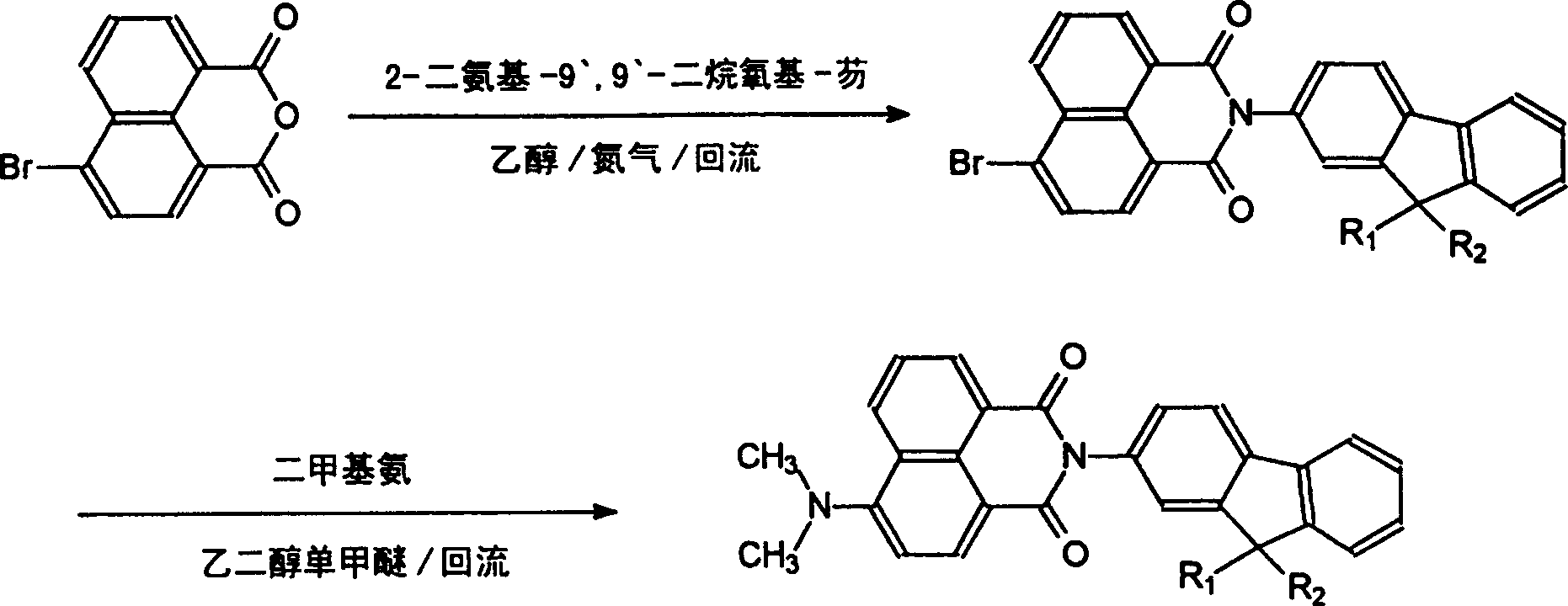
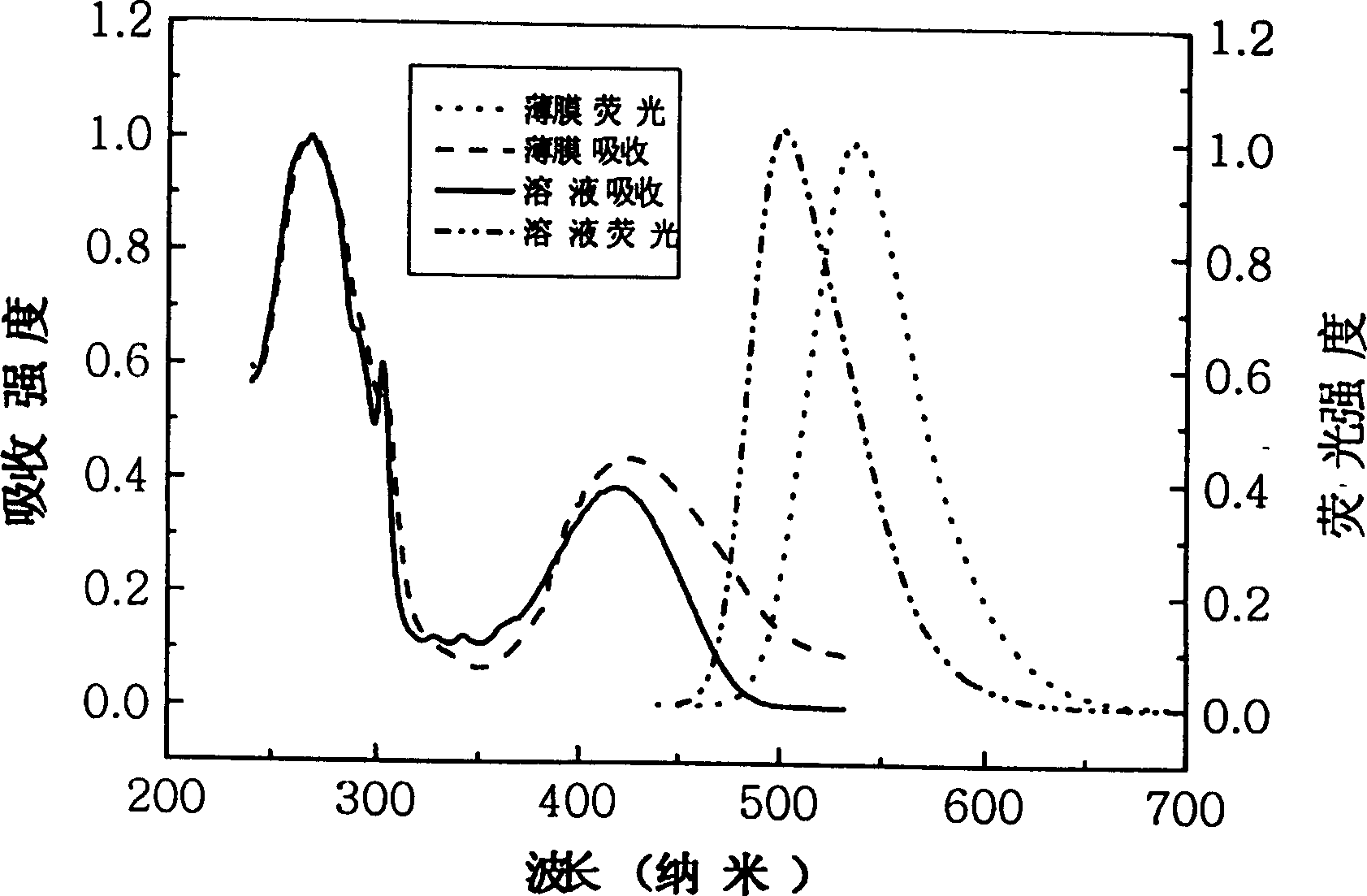
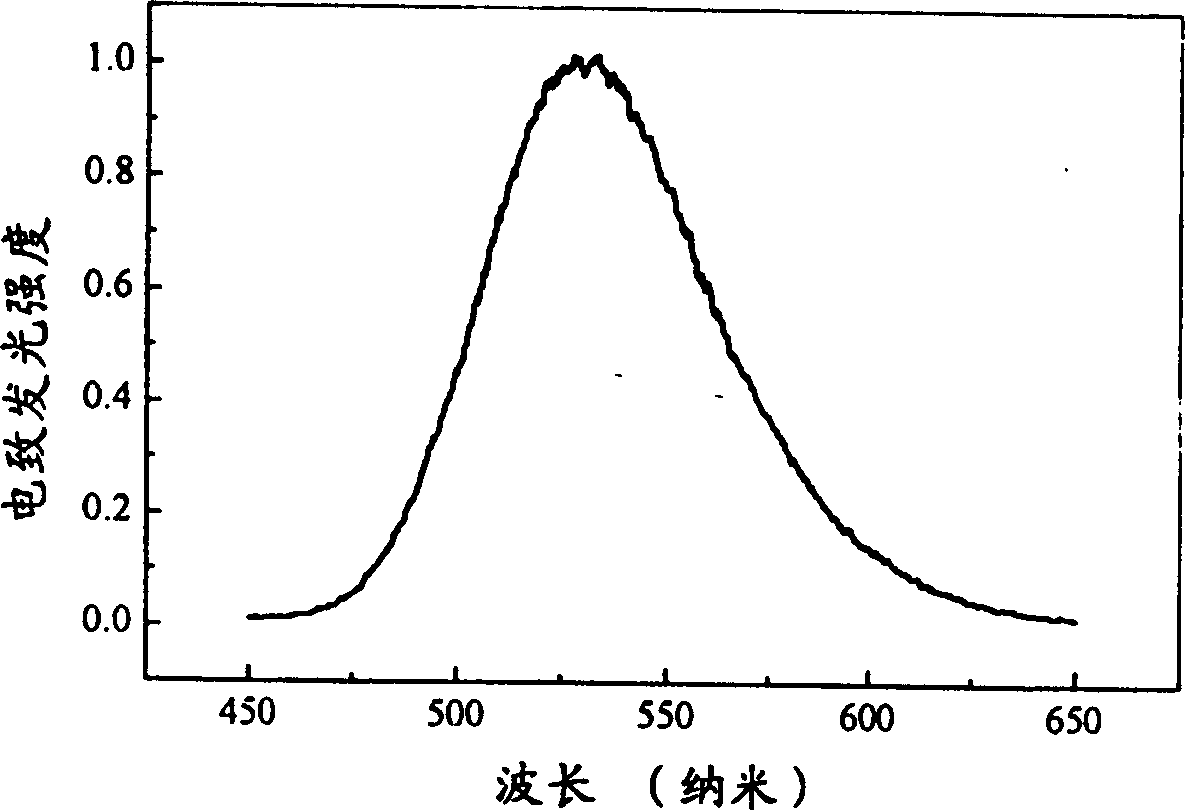
[0023] Synthetic route such as figure 1 shown.
[0024] (1) 4-bromo-N-(2'-fluorene)-1,8-naphthalene diimide (abbreviated as BFN): add 98% 4-bromo-1,8-naphthalene dianhydride (1.39 g; 5mmol), 96% 2-aminofluorene (0.95g, 5mmol), evacuated and filled with nitrogen for 30min, injected 120mL of 95% ethanol, heated, stirred and refluxed under nitrogen atmosphere for 24h, filtered at room temperature, purified by column chromatography Yellow powder (2.09 g, yields: 95%). mp: 285-286°C, MS (EI, m / z): 439 (100%, M + -1), 441 (98%, M + +1).Anal.Calcd for:C 25 h 14 BrNO 2 (Molecular weight: 440.29): C, 68.20%; H, 3.20%; N, 3.18%; Br, 18.15%. Found: C, 68.10%; H, 3.15%; N, 3.04%; Br, 18.13%. 1 HNMR (DMSO-d 6 , 300Hz) δ: 4.02 (2H, s), 7.38-7.45 (3H, m), 7.61-7.66 (2H, m), 7.98-8.01 (1H, d), 8.04-8.07 (2H, m), 8.29- 8.31(1H, d), 8.38-8.41(1H, d), 8.62-8.67(2H, t).FT-IR(KBr, cm -1): 1711, 1666, 1589, 1505, 1457, 1402, 1364, 1239, 1193, 1133, 1045, 974, 901, 826, 781, 736, 421.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com