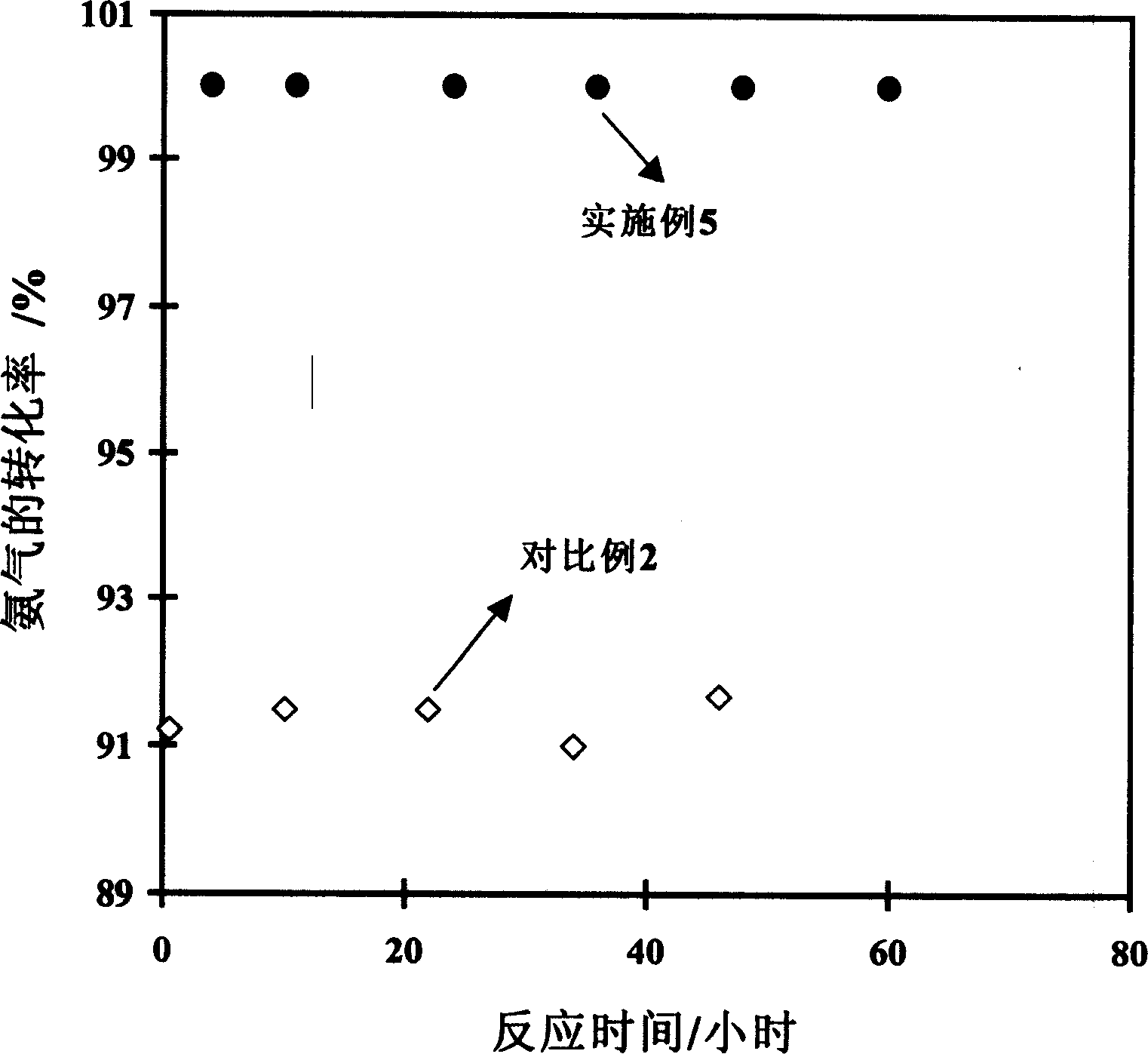
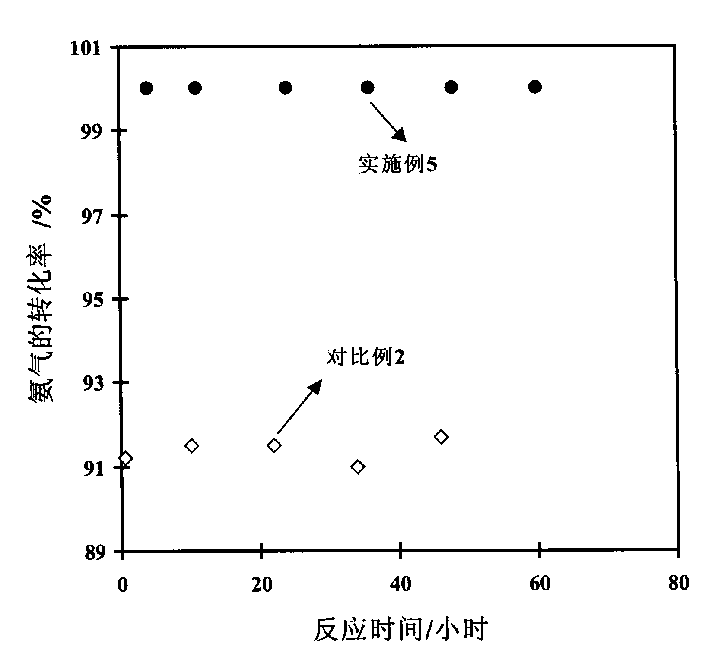
Low-temperature ammonia decomposition hydrogen preparation catalyst and preparing method thereof
A catalyst and ammonia decomposition technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low stack density of carbon nanotubes, high cost, and increased catalyst cost and other problems, to achieve the effect of simple preparation process and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take 0.1081g RuCl 3 Dissolve in 10ml of acetone to make a solution, and then mix with 1g of MgO [MgO is prepared according to the method described in CN 1280882A (2001), the grain size and specific surface of the sample after roasting at 600°C for 5 hours are 11nm and 150m 2 / g] mixed, stirred for 1 hour, dried at 55°C for 5 hours, then heated up to 600°C at 10°C / min, and fired at this temperature for 10 hours. After reduction of this catalyst at 500 °C, the Ru particle size is 4-10 nm. Get 0.1g catalyst (the content of Ru is 5%) and put in quartz reactor, under 25% H 2 -In the Ar atmosphere (80ml / min), the temperature was raised to 550°C at 5°C / min, and activated at this temperature for 2 hours, then the temperature was lowered to 500°C, and high-purity ammonia gas was introduced at this temperature for reaction. The flow rate is 50ml / min. Reaction results: the conversion rate of ammonia was 76.9%, and the generation rate of hydrogen was 25.7mmol / (min·g-cat).
Embodiment 2
[0024] The Ru / MgO catalyst was prepared according to the steps described in Example 1, then it was impregnated with NaOH aqueous solution, dried and calcined to obtain a NaRu / MgO catalyst with an atomic ratio of Ru and Na of 1:2. Get 0.1g catalyst and put in quartz reactor, in 25% H 2 -In the Ar atmosphere (80ml / min), the temperature was raised to 550°C at 5°C / min, and activated at this temperature for 2 hours, then the temperature was lowered to 520°C, and high-purity ammonia gas was introduced at this temperature for reaction. The flow rate is 50ml / min. Reaction result: the conversion rate of ammonia is 100%, and the generation rate of hydrogen is 33.4mmol / (min·g-cat).
Embodiment 3
[0026] Take 0.2162g RuCl 3 Dissolved in 10ml acetone to form a solution, and then mixed with 1g ZrO 2 【ZrO 2 Prepared according to the method described in CN 1260324A (2000), the grain size and specific surface of the sample after calcination at 600°C for 5 hours are 10nm and 70m 2 / g] mixed, stirred for 1 hour, dried at 55°C for 5 hours, then raised to 500°C at 10°C / min, and fired at this temperature for 10 hours. After reduction of this catalyst at 500 °C, the Ru particle size is 4-20 nm. Get 0.1g catalyst (the content of Ru is 9.8%) and put in the quartz reactor, under 25% H 2 -In the Ar atmosphere (80ml / min), the temperature was raised to 550°C at 5°C / min, and activated at this temperature for 2 hours, then the temperature was lowered to 500°C, and high-purity ammonia gas was introduced at this temperature for reaction. The flow rate is 50ml / min. Reaction results: the conversion rate of ammonia was 92.8%, and the generation rate of hydrogen was 31.0 mmol / (min·g-cat). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com