Microflow controlled chip flow-type biochemical analysis instrument and method for detecting biochemical components
A microfluidic chip and biochemical analyzer technology, applied in the field of biochemical analyzers, can solve the problems of unstable labeled microspheres, tailing emission peaks, asymmetric peak shapes, etc., and achieves light weight, fast response speed, and reduced size. Effect of Instrument Volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 further illustrates structure and working process of the present invention in conjunction with accompanying drawing
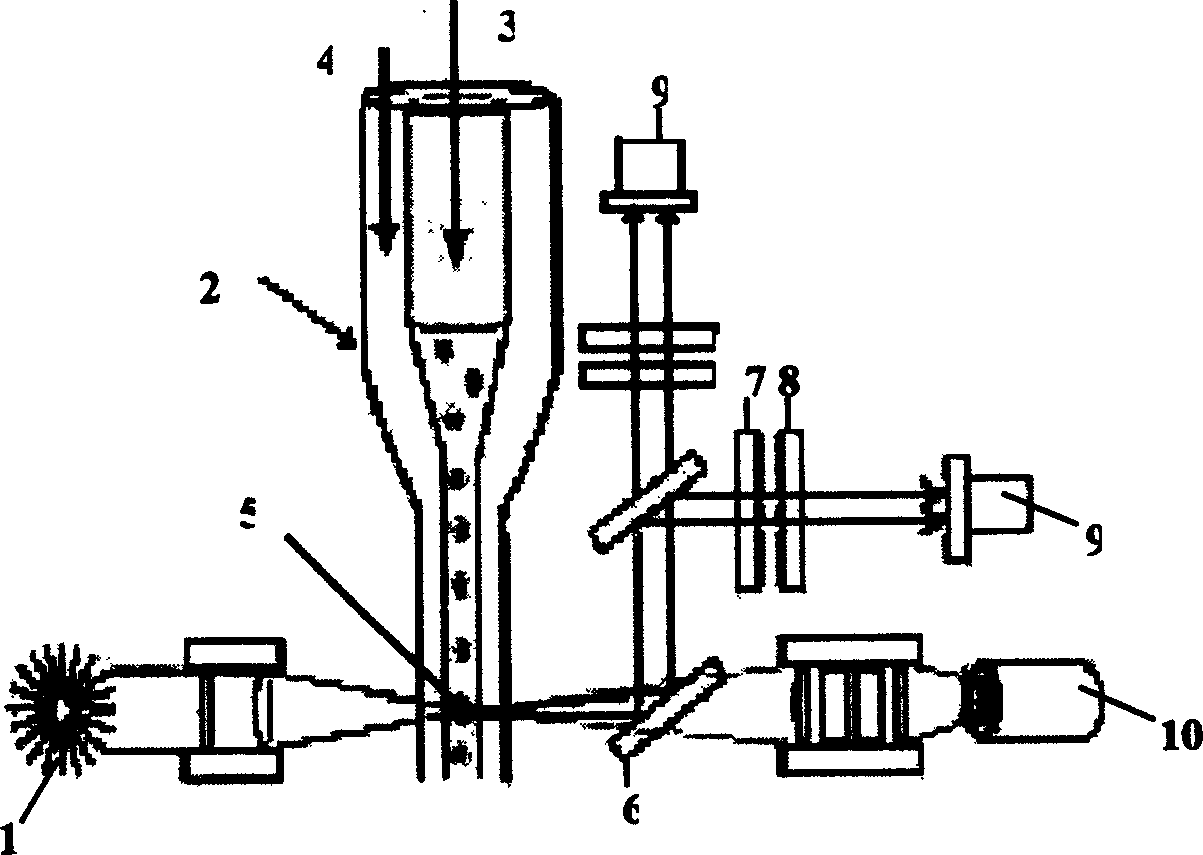
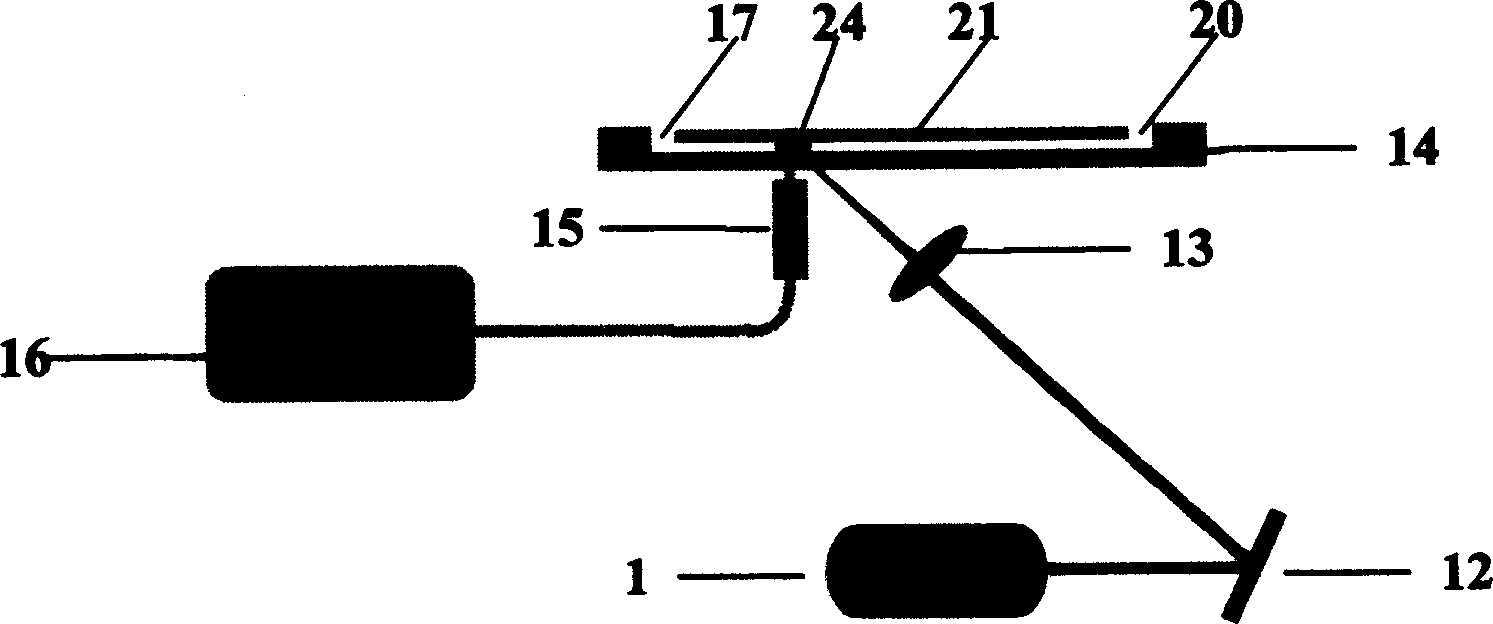
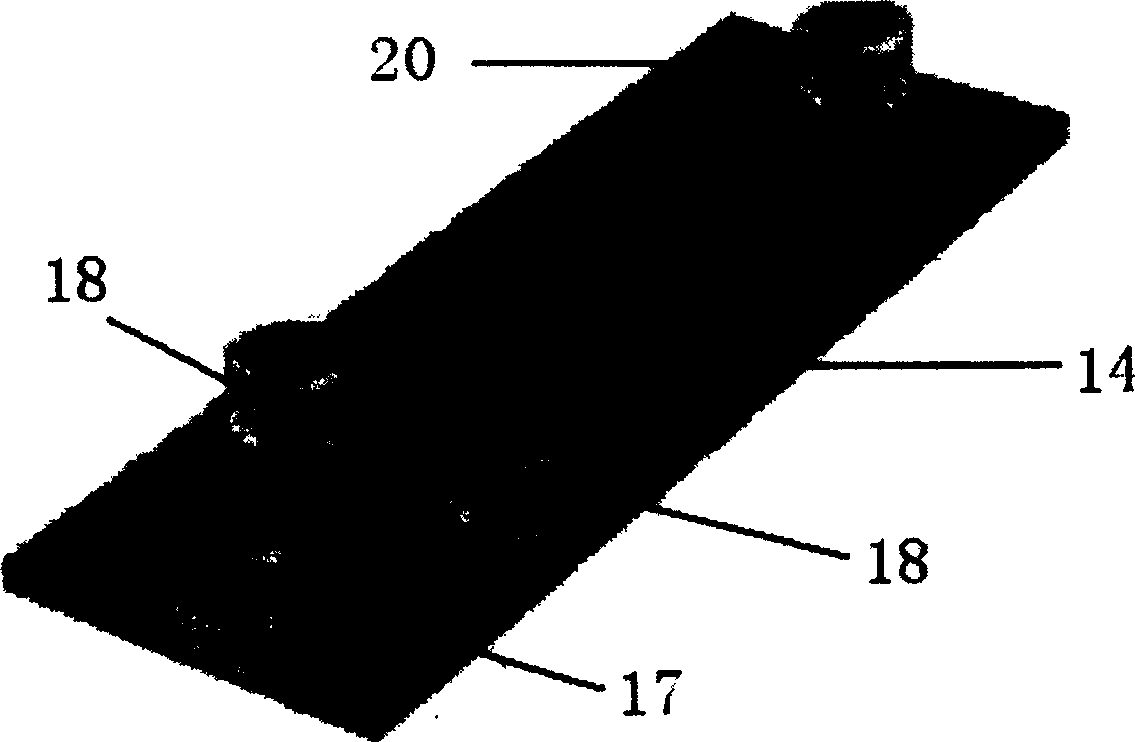
[0025]In the accompanying drawings, 1 is a laser light source, preferably a blue semiconductor laser; 12 is a plane mirror; 13 is a convex lens; 14 is a microfluidic chip, in which there are two cross channels with a width of several microns to more than one hundred microns 21. At the four end points of the microchannel 21, there are sample inflow holes 17, two opposite buffer solution inflow holes 18, and waste liquid outflow holes connected to the outside world. Sample inflow holes 17, buffer solution inflow holes 18, and waste liquid outflow Corresponding liquid reservoirs are respectively bonded on the holes 20, and an electrode is inserted in each of them; 24 is a detection area, which is located on the channel 21 leading to the waste liquid outflow hole 20; The detection point 24 is opposite, and the other end is closely connected with...
Embodiment 2
[0033] Example 2 Detecting the antigen contained in the sample:
[0034] 1. Shake the coded microspheres in a vortex shaker and an ultrasonic shaker to suspend them evenly; 3-(3-Dimethylaminopropyl) carbodiimide salt (EDC) was mixed and shaken, and left at room temperature for 20 minutes to activate the carboxyl groups on the surface of the microspheres.
[0035] 2. The activated microspheres were washed 3 times with PBS solution, then suspended by vortex, and the capture antibody was added quickly, after mixing, shake and incubate at room temperature for 30min, centrifuge at 200g for 15min, and discard the supernatant.
[0036] 3. Suspend the microspheres in BSA containing 1mg / mL and 0.02% Tween 20, incubate at 4°C for 30min, centrifuge at 200g for 15min, discard the supernatant; wash the microspheres twice with the above buffer, and suspend them in Incubate in 1 mL of cell culture medium at 4°C for 20 min; count with a hemocytometer, and adjust the concentration of suspende...
Embodiment 3
[0040] Example 3 Detection of cytokines by microsphere-labeled receptors
[0041] 1. The process of activating the hydroxyl groups on the surface of the microspheres is the same as in Example 2.
[0042] 2. Add receptors such as IL-1, 2, 6, and 12 to each microsphere, each microsphere corresponds to a receptor, mix and incubate at room temperature for 30 minutes, centrifuge at 200g for 15 minutes, and discard the supernatant ;
[0043] 3. Suspend the microspheres in BSA containing 1mg / mL and 0.02% Tween 20, incubate at 4°C for 30min, centrifuge at 200g for 15min, discard the supernatant; wash the microspheres twice with the above buffer, and suspend them in Incubate in 1 mL of cell culture medium at 4°C for 20 min; count with a hemocytometer, and adjust the concentration of suspended microspheres to 2×10 6 / mL, and placed in a dark place at 4°C;
[0044] 4. Add 100 μL of the solution containing the cytokine to be tested, incubate at 4°C for 45 minutes; centrifuge at 200 g f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com