Internediate for preparing quetiapin and preparation method of the intermediate
A derivative, piperazine technology, applied in the field of new intermediates, can solve problems such as uneconomical, reduced yield, and polluted final products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Preparation of formula IV starting compound
[0066] Phenyl 2-phenylthiophenylcarbamate
[0067] 20.13 g (0.1 mole) of 2-aminodiphenyl sulfide was dissolved in 250 ml of dichloromethane, and the resulting solution was cooled to 5°C. Half of the solution in which 18.79 g (15.1 ml, 0.12 moles) of phenyl chloroformate was dissolved in 26 ml of dichloromethane was slowly added to the above stirred 2-aminodiphenyl sulfide solution, and then, the chloroformic acid The other half of the phenyl ester solution was added simultaneously with a solution of 3.0 g (0.075 mol) sodium hydroxide and 9.2 g (0.0875 mol) sodium carbonate in 50 ml of water, taking care that the internal temperature did not exceed 10°C. After addition of the above solution, the reaction mixture was stirred at room temperature for 3 hours, the organic phase was separated, washed 3 times with a total of 250 ml of dilute hydrochloric acid, dried over anhydrous magnesium sulfate and evaporated. The residue was...
Embodiment 1
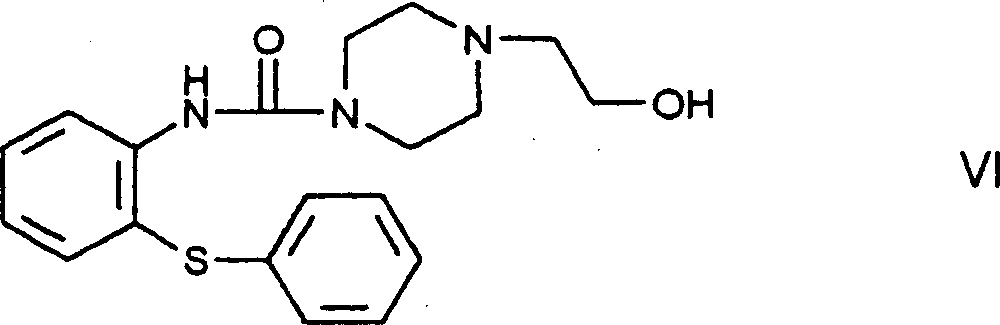
[0074] N-[4-(2-hydroxyethyl)piperazine-1-carbonyl]-2-aminodiphenylsulfide-compound of formula VI
[0075] 32.1g (0.1 moles) of phenyl 2-phenylthiophenylcarbamate were dissolved in 600ml of toluene, and then 13.0g (0.1 moles) of 1-(2-hydroxyethyl)piperene was added to the solution under stirring Zinc. The reaction mixture was allowed to stir at boiling temperature for 2 hours, then cooled to room temperature, washed with 600 ml of 1N sodium hydroxide solution and then twice with 200 ml of water each time. The organic phase was dried over anhydrous magnesium sulfate and evaporated. The residue was recrystallized from a 10:1 mixture of n-hexane-ethyl acetate, filtered, washed with n-hexane and dried.
[0076] This gave 33.9 g (94.8%) of the title compound in the form of white crystals. Melting point: 96-98°C.
[0077] Analysis: C 19 h 23 N 3 o 2 S(357.478)
[0078] Calculated: C 63.84%, H 6.49%, N 11.75%, S 8.97%;
[0079] Found values: C 63.57%, H 6.52%, N 11.71%, S 9....
Embodiment 2
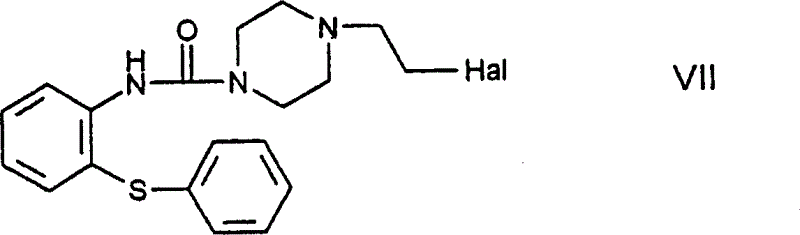
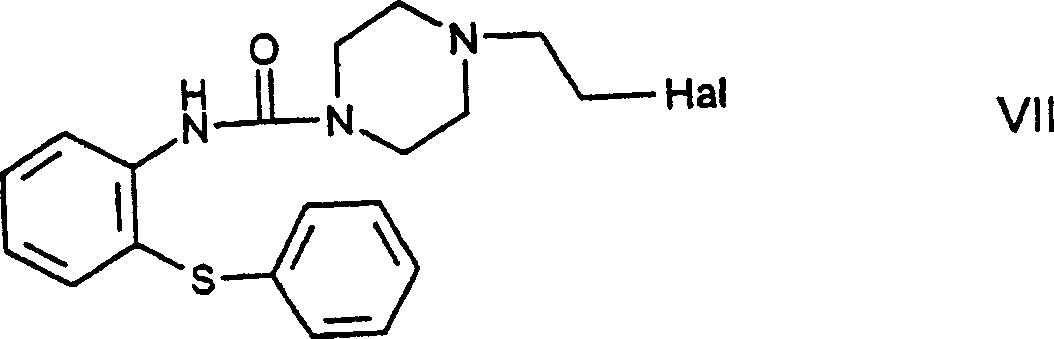
[0081] N-[4-(2-chloroethyl)piperazine-1-carbonyl]-2-aminodiphenyl sulfide-formula VII title compound
[0082] 18.8g (0.05mol) N-[4-(2-hydroxyethyl)piperazine-1-carbonyl]-2-aminodiphenylsulfide was heated under reflux in 65ml of thionyl chloride for 15 minutes, then evaporated, leaving The compound was crystallized from n-hexane. 18.5 g (89.7%) of product were obtained as the hydrochloride salt of the title compound.
[0083] Melting point: 180-183°C.
[0084] Base formation:
[0085] 2.78g (0.0275 moles) of triethylamine were added to 10.31g (0.025 moles) of the above-mentioned hydrochloride in 250ml of isopropanol solution, the reaction mixture was stirred at room temperature for 1 hour, poured into water, and washed with dichloromethane Extracted, dried over anhydrous magnesium sulfate and evaporated. This gave 8.0 g (85.1%) of the title compound.
[0086] Salts with benzenesulfonic acid
[0087] A solution of 3.48 g (0.022 mol) of benzenesulfonic acid in 10 ml of etha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com