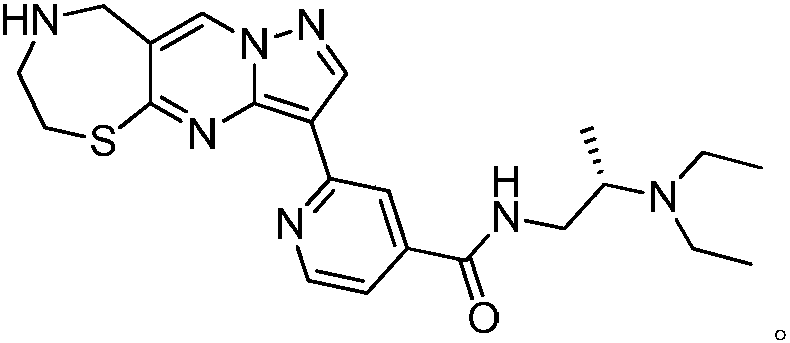
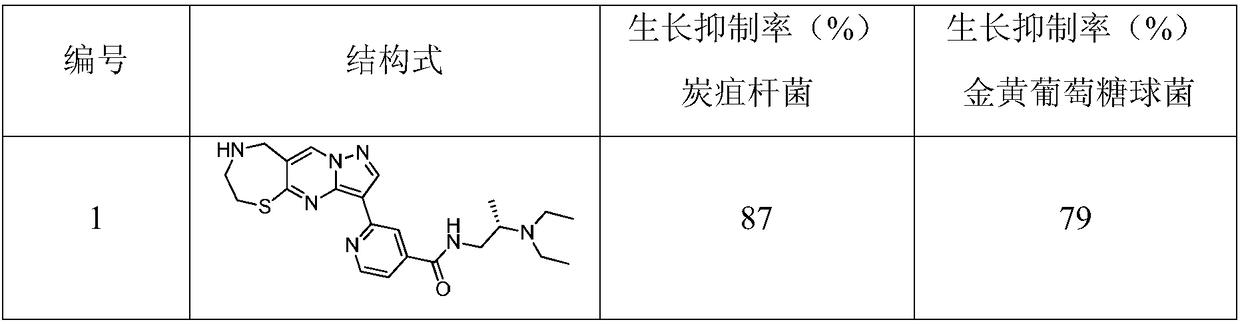
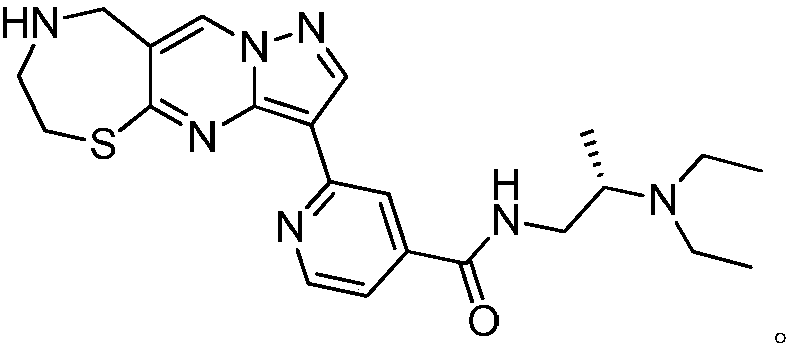
1,4-thiazepine medicinal compound for nursing skin ulcer as well as preparation method and application thereof
A technology of pharmaceutical compounds and thiazepines, which can be used in drug combinations, skin diseases, pharmaceutical formulations, etc., and can solve problems such as dressing bandages and ulcer wound adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Under nitrogen protection, the compound Add 150g (1mol) into 2000mL of N.N-dimethylformamide, cool the reaction temperature to 10°C and add 65g of N-chlorosuccinimide in three batches, a total of 195g (1.5mol), return to React at room temperature for 12 hours, LCMS monitors that the raw materials have reacted completely, then add 10,000 mL of ethyl acetate, stir for 10 minutes, wash the reaction solution with saturated sodium chloride solution until the organic phase does not contain N,N-dimethylformamide, and wash the organic phase with anhydrous sulfuric acid Dry 300g of magnesium, add 1000mL of n-hexane after concentration, stir and beat at 0°C, filter with suction and dry to obtain the compound 200g, the yield is 87.3%, – HRMS ((+)-ESI): m / z=231.0971 (calcd.231.0975for C 6 h 5 BrN 3 S,[M+H] + ).
Embodiment 2
[0026] In the reaction vial, put the compound 35g (0.15mol), 17.4g (0.15mol) of 2-chloroethylamine and 8g (0.2mol) of sodium hydride with a content of 60wt% were added to 100mL of tetrahydrofuran, and the gas in the reaction system was replaced with nitrogen three times, then heated to reflux for 10h, TLC After monitoring the complete reaction of the raw materials, it was lowered to room temperature, and 200 mL of dichloroethane was added to the reaction system, and the reaction solution was filtered after stirring for 10 minutes. After the filtrate was concentrated under reduced pressure, 100 mL of water was added, and 20 mL of hydrochloric acid solution was added. The mixed solution of ethyl acetate and n-hexane was extracted 2 times with 100mL of the reaction liquid, the water phase was adjusted to pH 11 by saturated sodium hydroxide solution, and then extracted 3 times with 100mL of chloroform, the organic phases were combined, dried with anhydrous sodium sulfate and then ...
Embodiment 3
[0028] In the reaction vial, put the compound Add 35g (0.15mol), 17.4g (0.15mol) of 2-chloroethylamine and 8g (0.2mol) of potassium hydride into 100mL of tetrahydrofuran, replace the gas in the reaction system with nitrogen for three times, then heat and reflux for 10h, and monitor the reaction of raw materials by TLC. Cool down to room temperature, add 200 mL of dichloroethane to the reaction system, stir for 10 minutes, and filter the reaction solution. After the filtrate is concentrated under reduced pressure, add 100 mL of water, then add 20 mL of hydrochloric acid solution, and use ethyl acetate and n-hexane with a volume ratio of 1:1 100 mL of the mixed solution of alkanes was used to extract the reaction solution twice, the aqueous phase was adjusted to pH 11 by saturated sodium hydroxide solution, and then extracted three times with 100 mL of chloroform, the organic phases were combined, dried with anhydrous sodium sulfate, and then concentrated to obtain the compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com