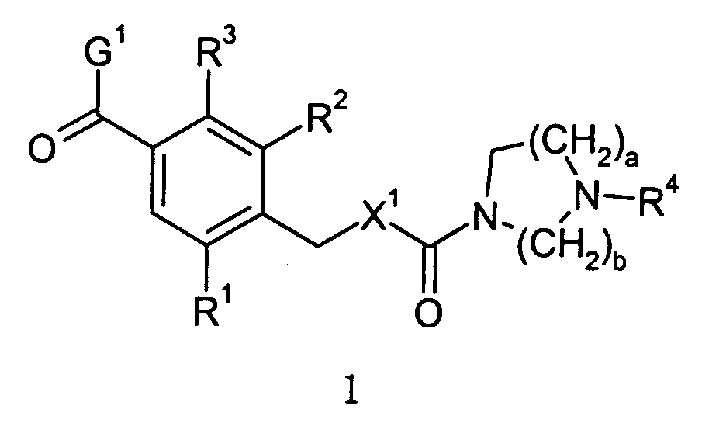
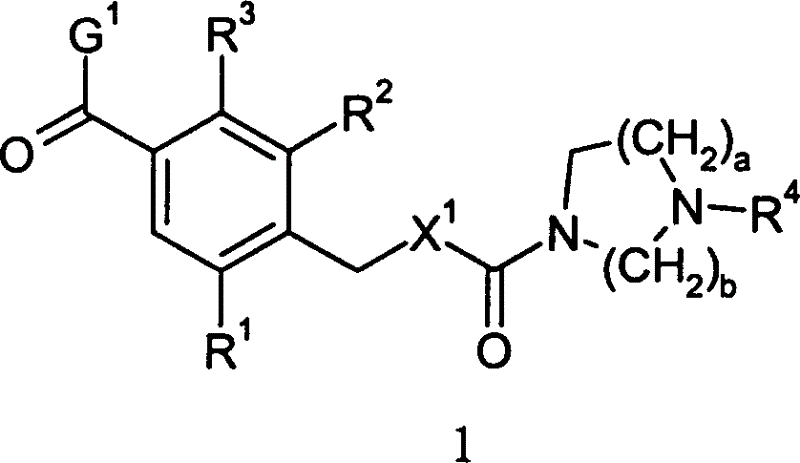
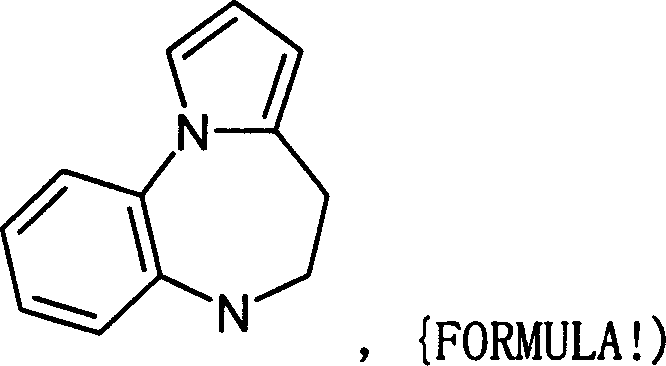
Diazacycloalkanes as oxytocin agonists
A technology of general formula and compound, applied in the field of non-peptide oxytocin agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] 1-Benzyl-4,10-dihydropyrazolo[5,4-b][1,5]benzodiazepine
[0154]
[0155] 1A: Ethyl 5-amino-1-benzylpyrazole-4-carboxylate
[0156] Benzylhydrazine dihydrochloride (4.29g, 22mmol) was added to ethyl (ethoxymethylene)cyanoacetate (3.38g, 20mmol) and triethylamine (6.15ml, 44mmol, 2eq) in ethanol ( 40 ml) solution, the mixture was heated at reflux for 18 hours. The solvent was removed in vacuo and the residue was purified by silica gel column chromatography (eluent 60% pet. ether / 40% ethyl acetate) to give a light yellow solid identified as 5-amino-1-benzylpyrazole-4-carboxy Acetate ethyl ester (4.3 g, 88%).
[0157] 1B: 1-Benzyl-5-(2′-nitrophenylamino)pyrazole-4-carboxylic acid ethyl ester
[0158] Sodium hydride (60% dispersion in oil, 520 mg, 13 mmol) was added portionwise to ethyl 5-amino-1-benzylpyrazole-4-carboxylate (2.2 g, 9 mmol) in anhydrous THF at 0 °C (30ml) solution. The mixture was warmed to room temperature and stirred for 2 hours, then 1-fluoro-2-n...
Embodiment 2
[0170] 1-Methyl-4,10-dihydropyrazolo[4,5-c]pyrido[2,3-b][1,4]diazepine
[0171]
[0172] 2A: Ethyl 1-methyl-2-(3′-nitro-2′-pyridylamino)pyrazole-4-carboxylate
[0173] Sodium hydride (60% dispersion in oil, 600 mg, 15 mmol) was added portionwise to ethyl 5-amino-1-methylpyrazole-4-carboxylate (1.69 g, 10 mmol) in anhydrous THF at 0 °C (15ml) suspension. The mixture was stirred at room temperature for 2 hours, then 2-chloro-3-nitropyridine (1.58 g, 10 mmol) was added and the resulting dark red suspension was stirred at room temperature for 18 hours. Join 1M KHSO 4 The reaction was quenched and the solvent was removed in vacuo. The residue was dissolved in ethyl acetate and the solution was washed with 0.3M KHSO 4 , saturated NaHCO 3 and washed with brine, in Na 2 SO 4Dry and concentrate in vacuo. The residue was purified by silica gel column chromatography (eluent 30% pet. ether / 70% ethyl acetate) to give 1-methyl-2-(3'-nitro-2'-pyridylamino)pyrazole- 4-Carboxylic a...
Embodiment 3
[0183] 4-Aminomethyl-3-chlorobenzoic acid tert-butyl ester
[0184]
[0185] 3A: tert-butyl 3-chloro-4-methylbenzoate
[0186] Thionyl chloride (11ml, 150mmol) was added to a suspension of 3-chloro-4-methylbenzoic acid (5.12g, 30mmol) in toluene (25ml), and the mixture was heated under reflux for 2 hours. The solvent was removed in vacuo and the residue was azeotroped three times with toluene, then dissolved in anhydrous THF (40ml) and cooled to 0°C. Lithium tert-butoxide (2.4 g, 30 mmol) was added, and the mixture was stirred at room temperature for 3 days. Water (5ml) was added and the solvent was removed in vacuo. The residue was dissolved in ethyl acetate. solution with 0.3M KHSO 4 , saturated NaHCO 3 and washed with brine, in Na 2 SO 4 Drying on ethylene oxide and concentration in vacuo afforded a pale yellow gum identified as tert-butyl 3-chloro-4-methylbenzoate (5.4 g, 79%).
[0187] 3B: tert-butyl 4-bromomethyl-3-chlorobenzoate
[0188] N-bromosuccinimide (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com