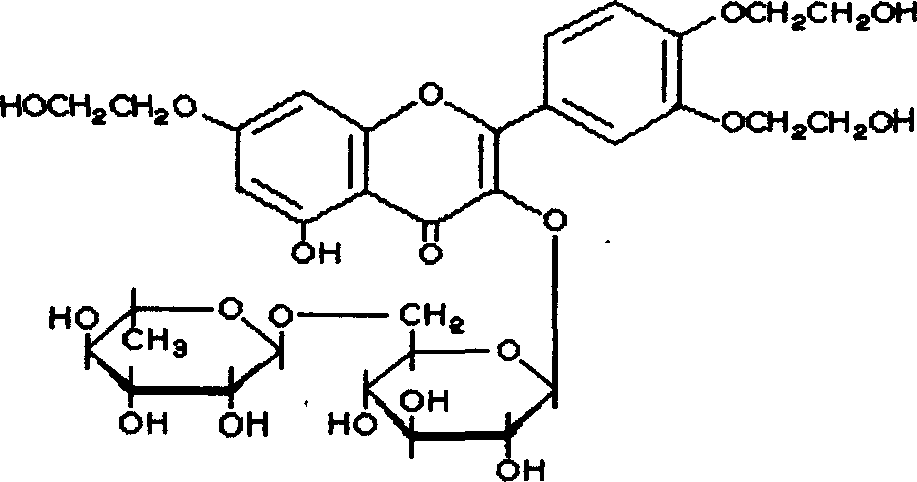
Quke rutin granule agent and its preparation method
A technology of granules and main drug, applied in the field of Troxerutin granules and preparation thereof, can solve the problems of inconvenience of taking high-content Troxerutin tablets, inability to treat venous insufficiency and hemorrhoids, and achieve lasting curative effect The effect of stabilizing, strengthening resistance, and enhancing venous tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Troxerutin granule, comprising principal agent and auxiliary material, is characterized in that being formulated into the granule that the principal agent content is 500g (in 1000 packs) by following percentage by weight: principal agent 45%, auxiliary material is cyclamate of 1% , 52% mannitol and 2% polyvinylpyrrolidone.
[0030] Troxerutin granule of the present invention is formulated from the raw materials of following weight ratio:
[0031] Troxerutin 500g
[0032] Cyclamate 11g
[0033] Mannitol 578g
[0034] Polyvinylpyrrolidone 22g
[0035] A total of 1000 packs were made
[0036] Wherein, cyclamate is a flavoring agent, mannitol is a filler, and polyvinylpyrrolidone is a binder.
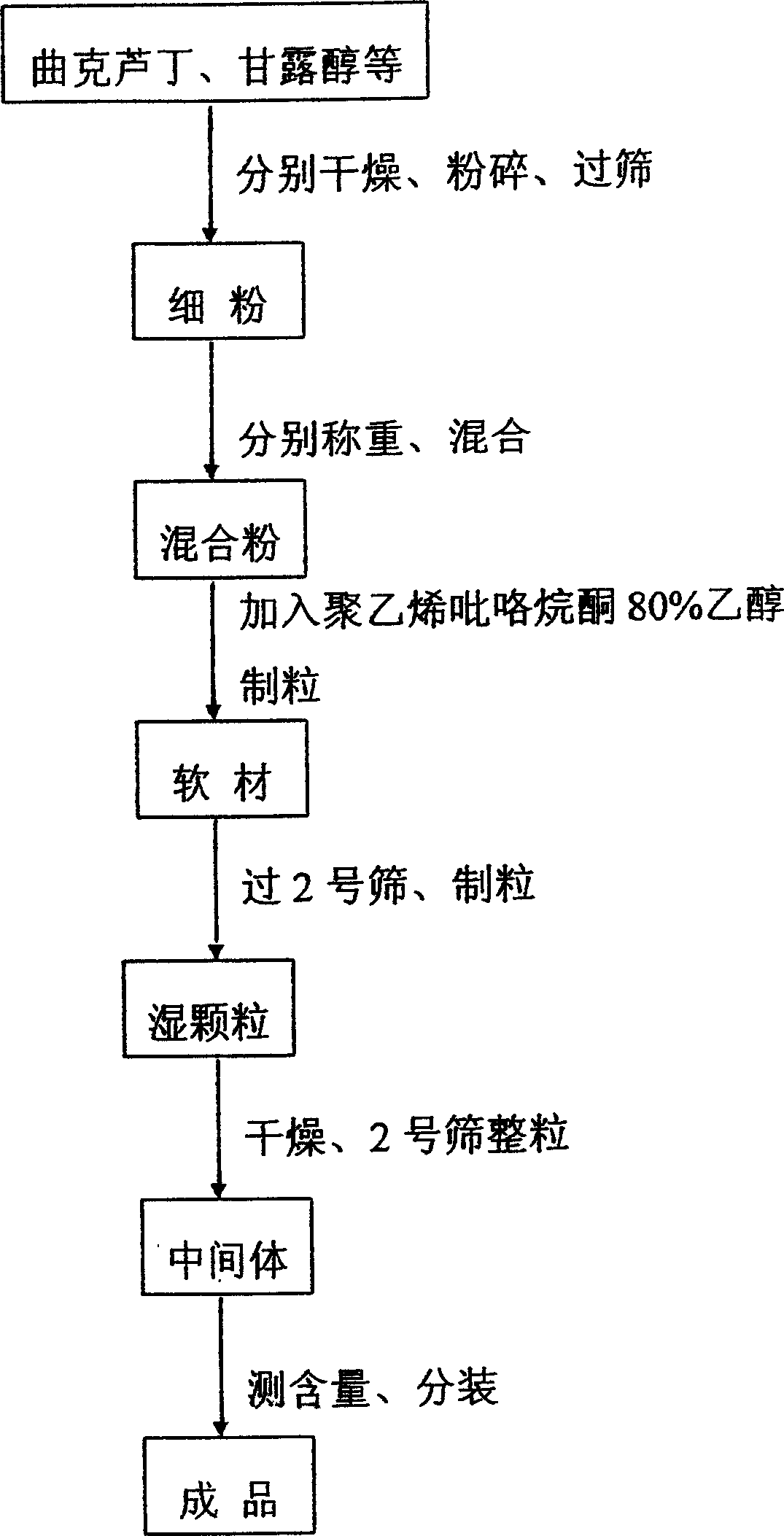
[0037] The preparation method of troxerutin granule of the present invention comprises the steps of drying, pulverizing, sieving, weighing, mixing, granulating, granulating, measuring and subpackaging, and is characterized in that: the specific process steps are as follows:
[...
Embodiment 2
[0046] Troxerutin granules, including main drug and auxiliary materials, are characterized in that the granules whose main drug content is 5000g (in 1000 bags) are formulated according to the following percentage by weight: main drug 55%, and auxiliary materials are 1% cyclamate , 43% mannitol and 1% polyvinylpyrrolidone.
[0047] Troxerutin granule of the present invention is formulated from the raw materials of following weight ratio:
[0048] Troxerutin 5000g
[0049] Cyclamate 91g
[0050] Mannitol 3910g
[0051] Polyvinylpyrrolidone 91g
[0052] A total of 1000 packs were made
[0053] Wherein, cyclamate is a flavoring agent, mannitol is a filler, and polyvinylpyrrolidone is a binder.
[0054] The preparation method of troxerutin granule of the present invention comprises the steps of drying, pulverizing, sieving, weighing, mixing, granulating, granulating, measuring and subpackaging, and is characterized in that: the specific process steps are as follows:
[0055] ...
Embodiment 3
[0063] Troxerutin granules, including main ingredients and auxiliary materials, are characterized in that they are formulated into granules whose main ingredient content is 3500g (in 1000 packs) according to the following weight percentage proportioning: main ingredient 50%, auxiliary ingredients 1% Cyclamate, 48% mannitol and 1% polyvinylpyrrolidone.
[0064] Troxerutin granule of the present invention is formulated from the raw materials of following weight ratio:
[0065] Troxerutin 3500g
[0066] Cyclamate 70g
[0067] Mannitol 3360g
[0068] Polyvinylpyrrolidone 70g
[0069] A total of 1000 packs were made
[0070] Wherein, cyclamate is a flavoring agent, mannitol is a filler, and polyvinylpyrrolidone is a binder.
[0071] The preparation method of troxerutin granule of the present invention comprises the steps of drying, pulverizing, sieving, weighing, mixing, granulating, granulating, measuring and subpackaging, and is characterized in that: the specific process st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com