Sibutramine aliphatic salt of organic acid, its preparation process and use
A technology of aliphatic organic acids and organic acid salts, applied in organic chemistry and other fields, can solve problems such as limited research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
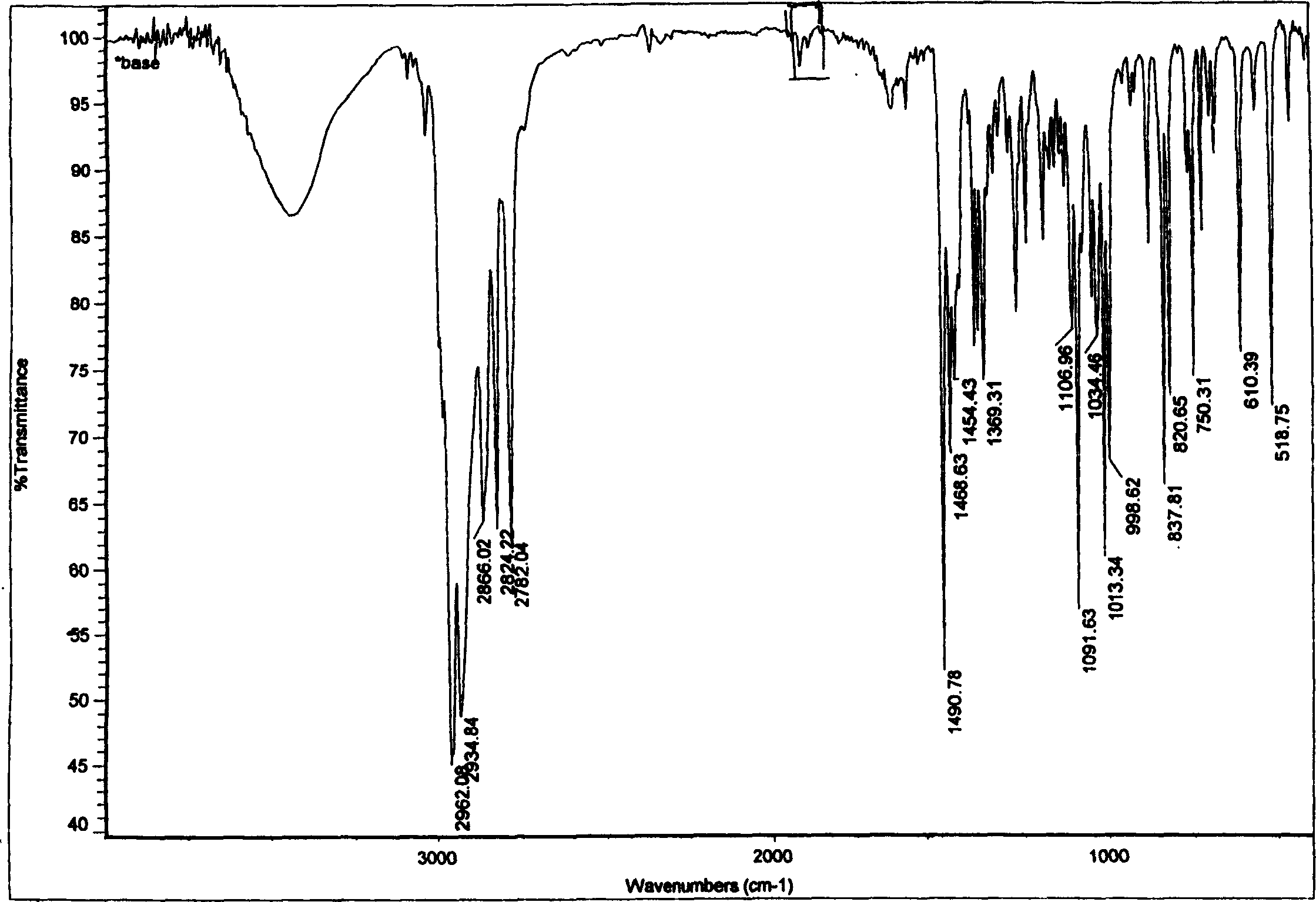
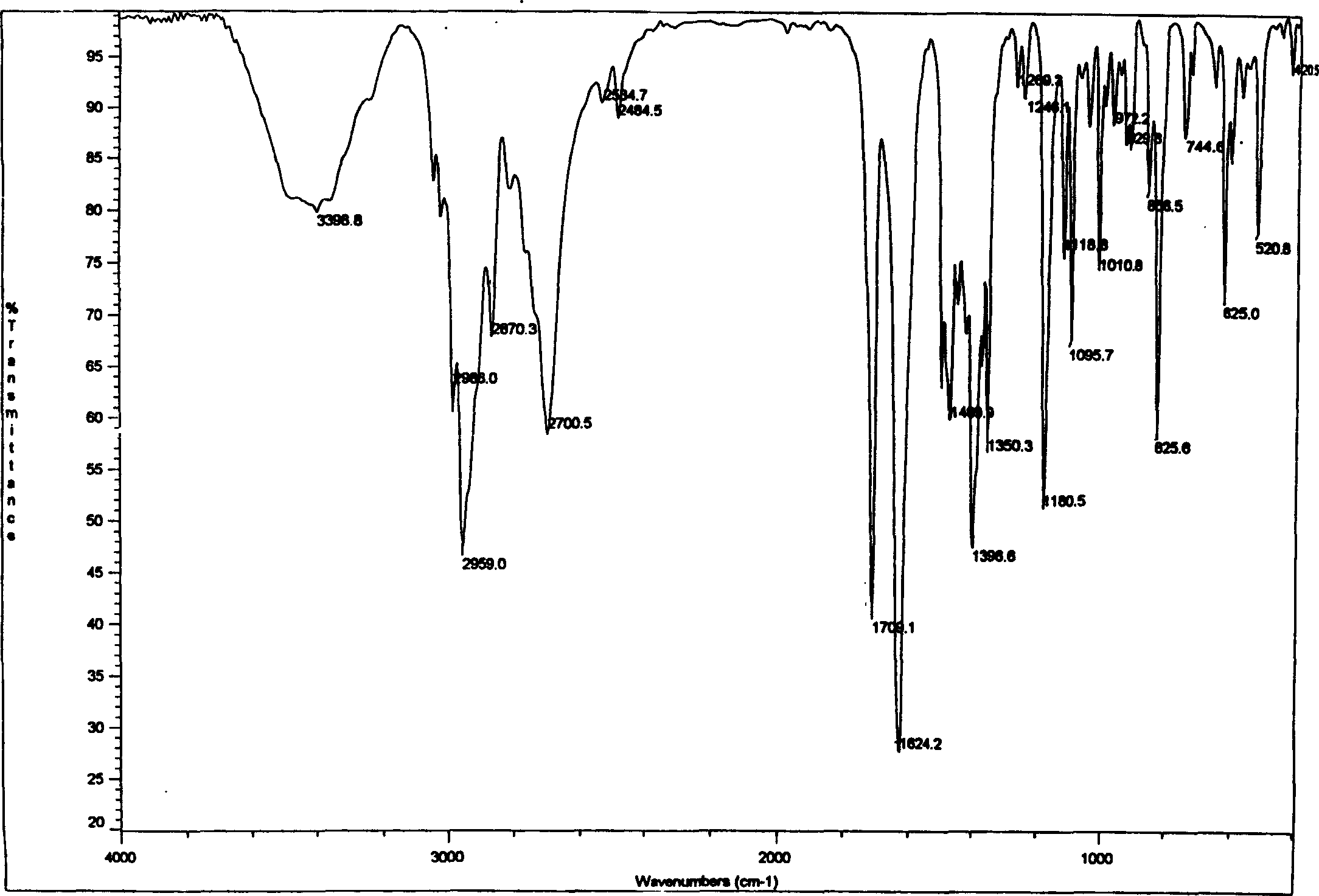
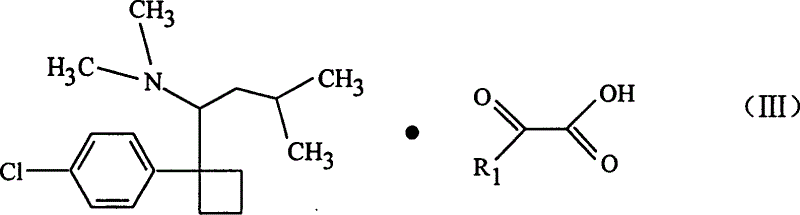
[0032] The present invention develops a new type of aliphatic organic acid salt of sibutramine on the basis of previous studies on sibutramine and its salts. The salt can play a more effective role in the prevention and treatment of diseases such as diabetes and obesity. Good results.
[0033] The invention uses sibutramine hydrochloride as the starting raw material, which is added to the caustic alkali aqueous solution, and the molar ratio of feeding is: hydrochloride: NaOH=1: 1~1: 8, and after a period of reaction, it is extracted with ether. Then dry and concentrate to obtain sibutramine free base as white solid. Then, sibutramine free base and pyruvic acid or an aliphatic organic acid similar to pyruvic acid were dissolved in an organic solvent to form a solution, and then the two organic solutions were mixed and stirred at room temperature, and reacted for 0.5 to 2 hours. After that, white crystals are precipitated, filtered and dried to obtain sibutramine aliphatic orga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com