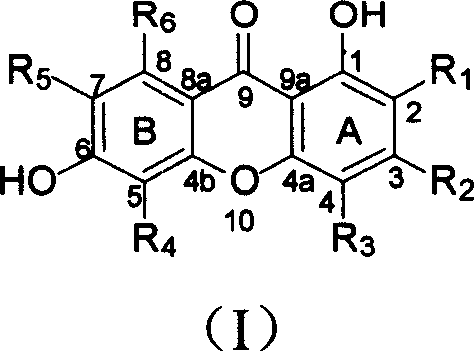
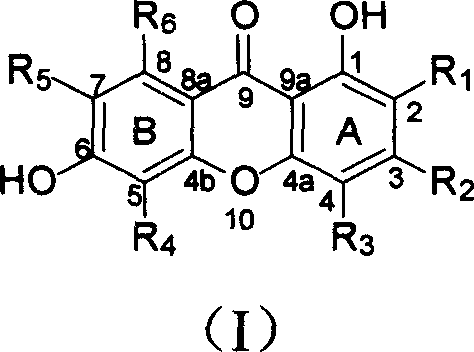
Isopentenyl xanthone compounds and their use in the preparation of antitumor medicines
A technology of prenyl ketone and tumor drug, applied in the preparation of anti-tumor drugs, natural anti-tumor drugs, the field of prenyl ketone compounds, can solve the problem of unknown anti-tumor active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Extraction and separation of new isopentenyl ketones from Zhe tree
[0043] Dry and crush 13.6kg of the root of C.tricuspidata (Carr.) Bur., extract with 95% ethanol, concentrate the extract under reduced pressure to obtain extract, suspend the extract with water, and extract with petroleum ether and chloroform in turn , to obtain petroleum ether part, chloroform part and water-soluble part. The chloroform part was subjected to silica gel column chromatography, followed by gradient elution with petroleum ether-acetone (20:1-5:5), and the fractions were collected. From petroleum ether-acetone 10:1 flow fraction, obtain citrus ketone B (2) 400mg; Petroleum ether-acetone 9: 1 fraction is subjected to repeated column chromatography to obtain citrus ketone F (6) 710mg and citrus ketone B (2) Talentone H (8) 167 mg; Petroleum ether-acetone 8: 2 fractions were subjected to repeated column chromatography to obtain tylone C (3) 33 mg, tylone D (4) 220 mg and tylone G(7...
Embodiment 2
[0060] Embodiment 2 Compounds of the present invention inhibit human tumor cell proliferation test in vitro
[0061] 1. Screening of SGC-7901, BGC-823, SMMC-7721, HCT cell proliferation
[0062] Using the modified MTT method, the tumor cells in logarithmic growth phase were added to a 96-well culture plate, 90 μL per well. Subsequently, 10 μL of test samples (0.31-20 μg / mL) in four concentrations were added to each well. Three replicate wells were set up for the sample addition group. cells at 37°C, 5% CO 2 After incubating in the incubator for 72 hours, add MTT solution 5 mg / mL, 10 μL. After continuing to cultivate for 4 hours, add triple solution [10% SDS-5% isobutanol-0.012mol / L HCl], 90 μL / well, and after 12 hours, measure the OD value of each well at a wavelength of 550 nm with a microplate reader.
[0063] Cell inhibition rate=(OD value of control group-OD value of medication group) / OD value of control group×100%. Calculate the IC of each sample with GWBASIC softwar...
Embodiment 3
[0067] Effects of Compounds of the Present Invention on Angiogenesis of Chicken Embryo Chorioallantoic Membrane (CAM)
[0068] experimental method:
[0069] (1) Put the eggs into the incubator at 37°C after being sterilized, with the air chamber upwards, and rotate 3-4 times a day. On the ninth day of hatching, after disinfecting the surface of the eggs, make a small hole of 1-2mm on the top of the air chamber, at a distance from the fetal head. Mark a rectangular area of 1.0cm×1.5cm on the projected part of the eggshell in the first 1cm and between the two yolk veins, grind and cut through the eggshell, and gently scratch a small hole with a diameter of about 1mm on the eggshell membrane, and add a little Separation of the eggshell membrane on the edge of the small hole with sterile pure water, put a sterile microporous filter membrane carrier with a diameter of 6mm on the least blood vessel of the CAM, and then add large, medium and small doses of the compound to be tested...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com