SARS coronavirus nucleocapsid protein monoclonal antibody, hybridoma for producing the same, detection agent containing the same and use thereof
A monoclonal antibody, nucleocapsid protein technology, applied in the field of diagnosing SARS, hybridoma, specific monoclonal antibody, to achieve the effect of good repeatability, accurate detection and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
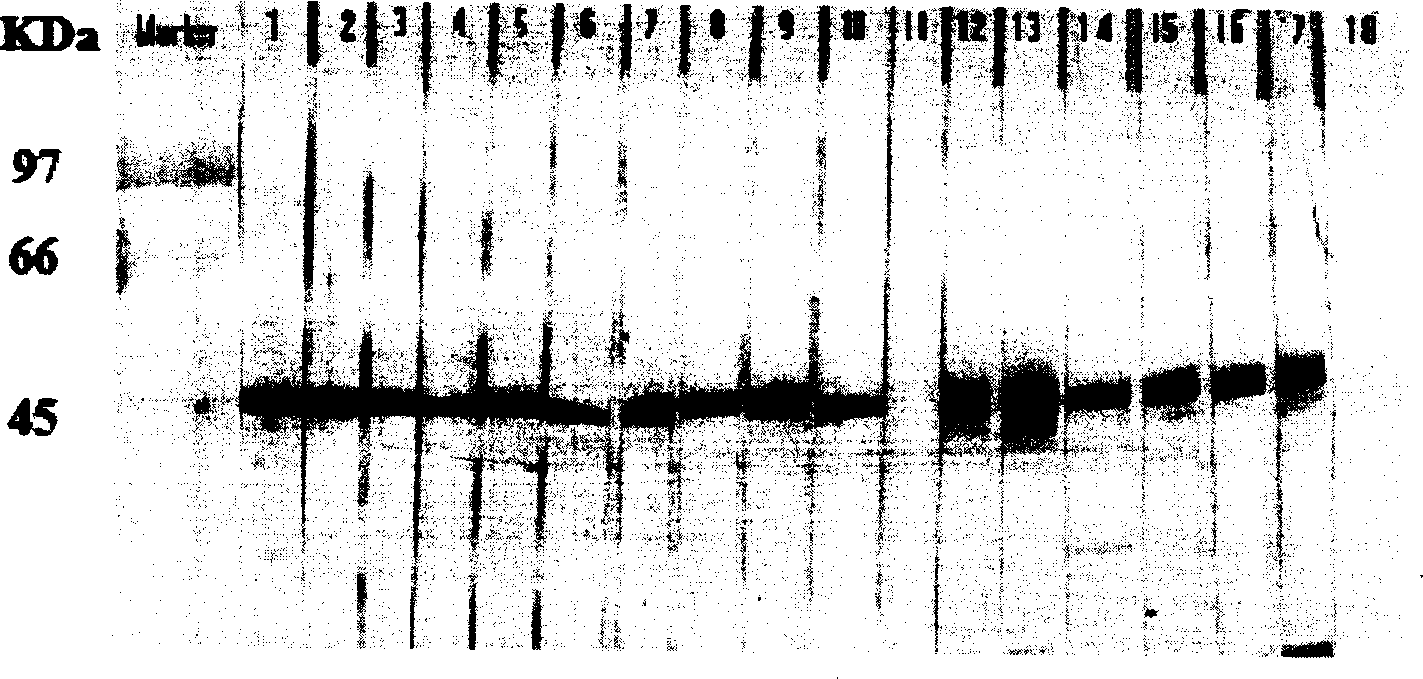
[0049] Embodiment 1: the preparation method of SARS-CoV nucleocapsid protein (hereinafter referred to as N protein) monoclonal antibody.
[0050] 1. Preparation of immune antigen
[0051] The immunogen used to prepare the monoclonal antibody in the present invention is a genetically recombined SARS-CoV N protein and an inactivated natural virus antigen, and the genetically recombined SARS-CoV N egg is an engineering strain carrying a SARS-CoV N protein gene. Escherichia coli strains were prepared. It was confirmed by sequencing that the nucleocapsid gene sequence of SARS coronavirus carried by the strain was consistent with the nucleocapsid gene sequence of SARS coronavirus HKU-39849 strain (or other strains) published by PUBMED. The preparation of recombinant SARS-CoV N protein is carried out according to conventional methods, and the N fusion protein antigen is obtained by purifying with nickel-nitrilotriacetic acid metal affinity chromatography. For detailed preparation me...
Embodiment 2
[0058] Embodiment 2: screening the hybridoma that secretes SARS-CoV nucleocapsid protein monoclonal antibody
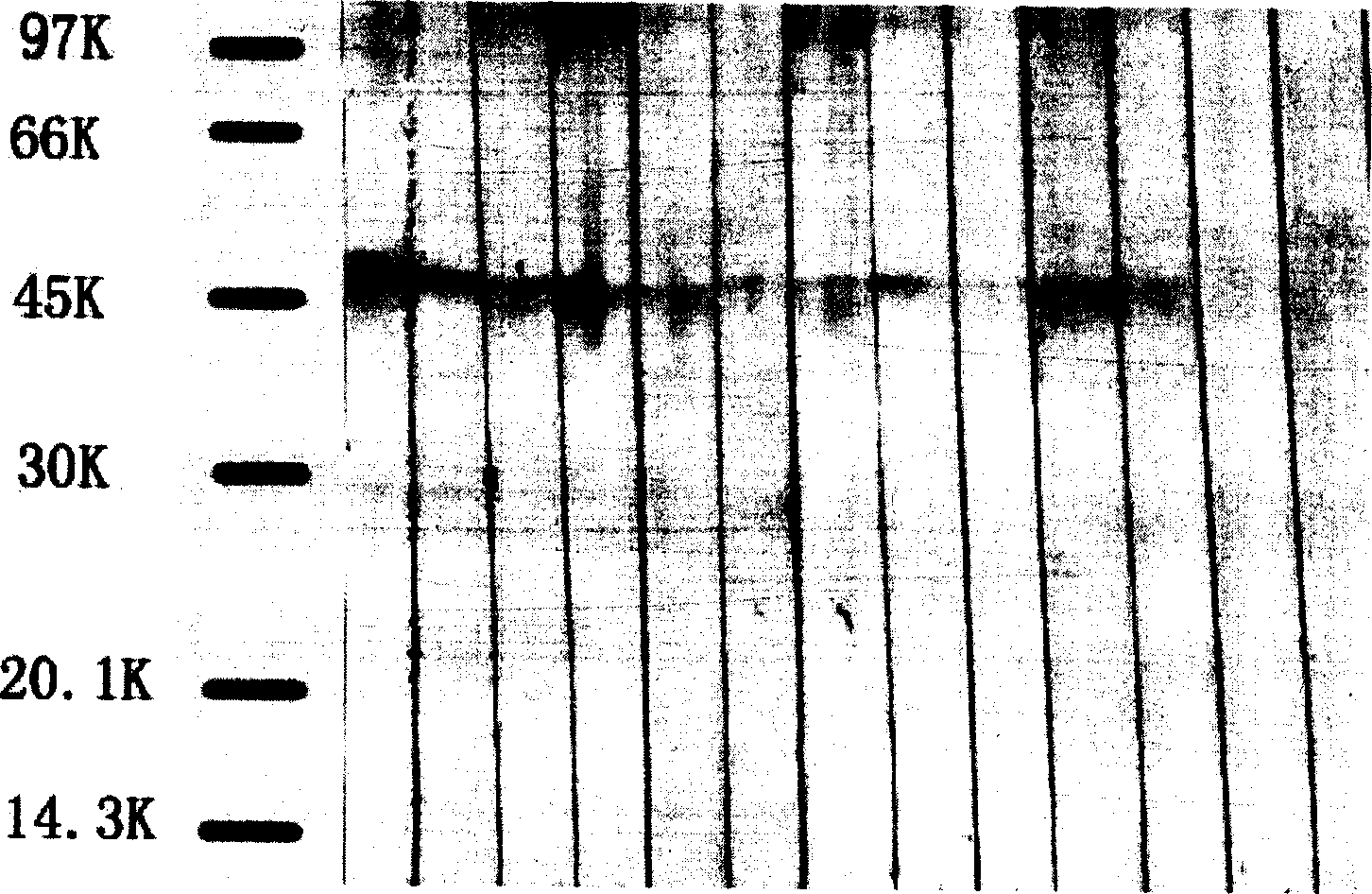
[0059]In order to detect the existence of antibody-producing clones, the method for measuring the titer of immune serum in Example 1, that is, the indirect ELISA method, is used to detect the cell culture supernatant. The brief description is as follows: the hybridoma culture supernatant is added to the coated well, 0.1 ml / Wells were kept at 37°C for 30 minutes. After washing the plate five times with washing solution, horseradish peroxidase-labeled goat anti-mouse IgG (ZYMED LABORATORIES, INC., USA) diluted 1:2000 was added, 0.1 ml / well at 37°C for 30 minutes. After washing the plate as above, add the substrate TMB, 0.1 ml / well, protect from light at room temperature for 10 minutes, add 0.1 ml / well 2M H 2 SO 2 Terminate the reaction and measure the absorbance at 450 nm. The strong positive clone hybridoma cells were selected for formal cloning, and the limiting di...
Embodiment 3
[0060] Example 3: SARS-CoV Nucleocapsid Protein Monoclonal Antibody Subclass Detection
[0061] The 4 clones obtained in this example were tested by the indirect ELISA method described in Example 1 to determine the antibody subclass they produced. Add hybridoma culture supernatant to coated wells, 0.1 ml / well at 37°C for 30 minutes, wash the plate five times with washing solution, add 1:1000 dilution of horseradish peroxidase-labeled rabbit anti-mouse different Subclass-specific immunoglobulins These antibodies include rabbit anti-mouse IgG1 (ZYMED LABORATORIES, INC, USA, Cat. No. 61-0120), rabbit anti-mouse IgG2a (supra, Cat. No. 61-0220), rabbit anti-mouse IgG2b ( Same as above, catalog number 61-0320), rabbit anti-mouse IgG3 (same as above, catalog number 61-0420), rabbit anti-mouse IgM (same as above, cat. After the plate, add substrate TMB, 0.1 ml / well, protect from light at room temperature for 10 minutes, add 0.1 ml / well 2M H 2 SO 2 Terminate the reaction and measure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com