Usage of differentiation protein of medullary cell MyD88 in preparing medicinal preparation
A technology of cell differentiation and pharmaceutical preparation, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
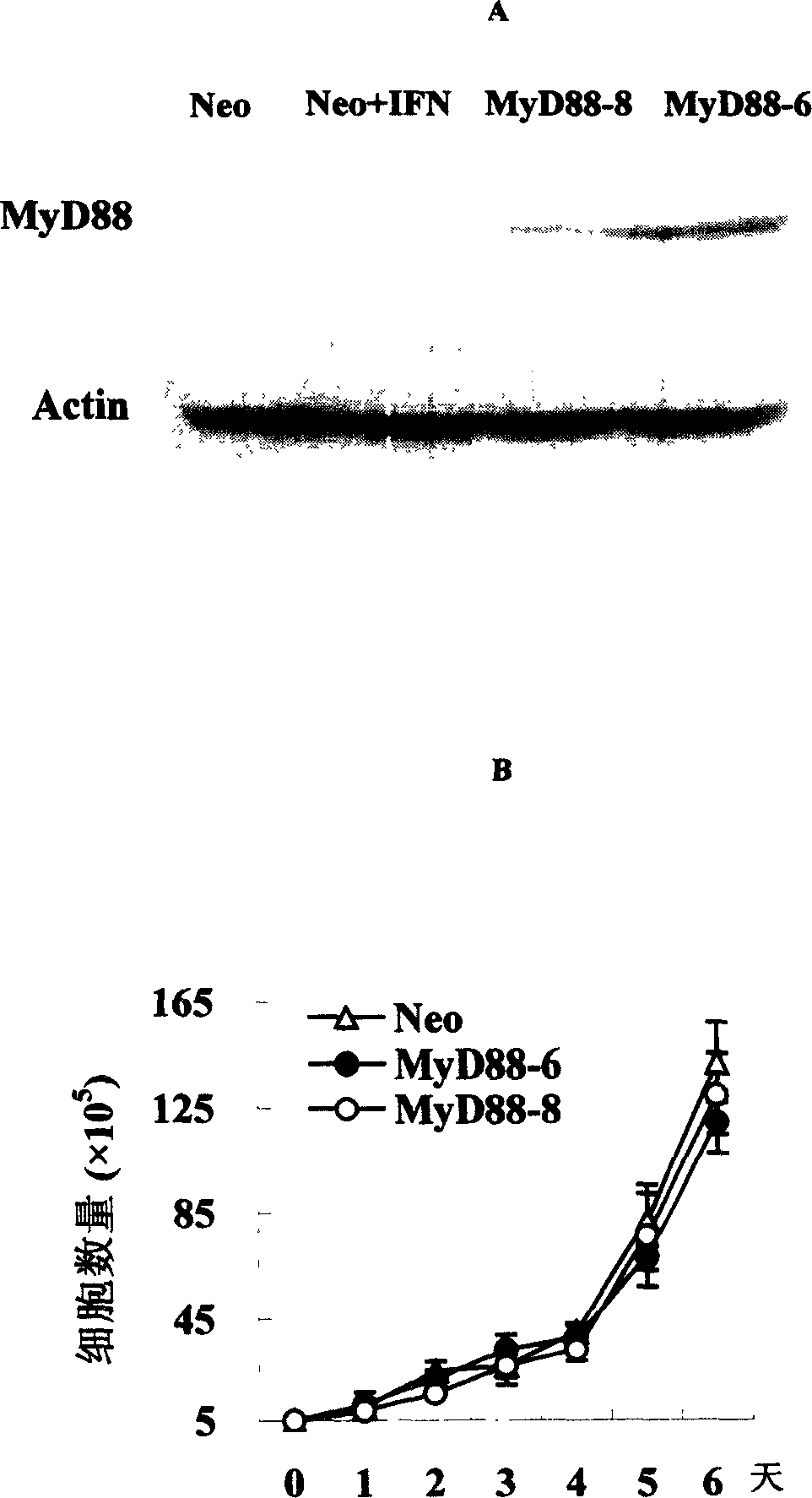
[0042] Example 1 Establishment of MyD88 Stable Expression Cell Line
[0043] Human liver cancer HepG2 cells were cultured in 10% fetal bovine serum, 10 5 u / L penicillin, 0.1g / L streptomycin in DMEM medium (Gibco). The empty vector pcDNA3.1 / myc-His or pcDNA-MyD88 (8 μg each) was transfected into HepG2 cells (1×10 6 After 36 hours, the cells were inoculated into selective medium containing 200 μg / ml G418 (Gibco Company) at a ratio of 1:4. After 3 weeks of selection by G418, single-cell clones resistant to neozyme were selected, and a neozyme-resistant control cell line (Neo) was selected from cells transfected with the empty vector pcDNA3.1 / myc-His , The cells transfected with pcDNA-MyD88 were selected to select two cell lines with stable expression of MyD88, namely MyD88-6 and MyD88-8. The MyD88 monoclonal antibody was used to detect the expression level of MyD88 protein in the above two MyD88 stable expression cell lines and Neo cell lines treated or untreated by IFN-α by W...
Embodiment 2
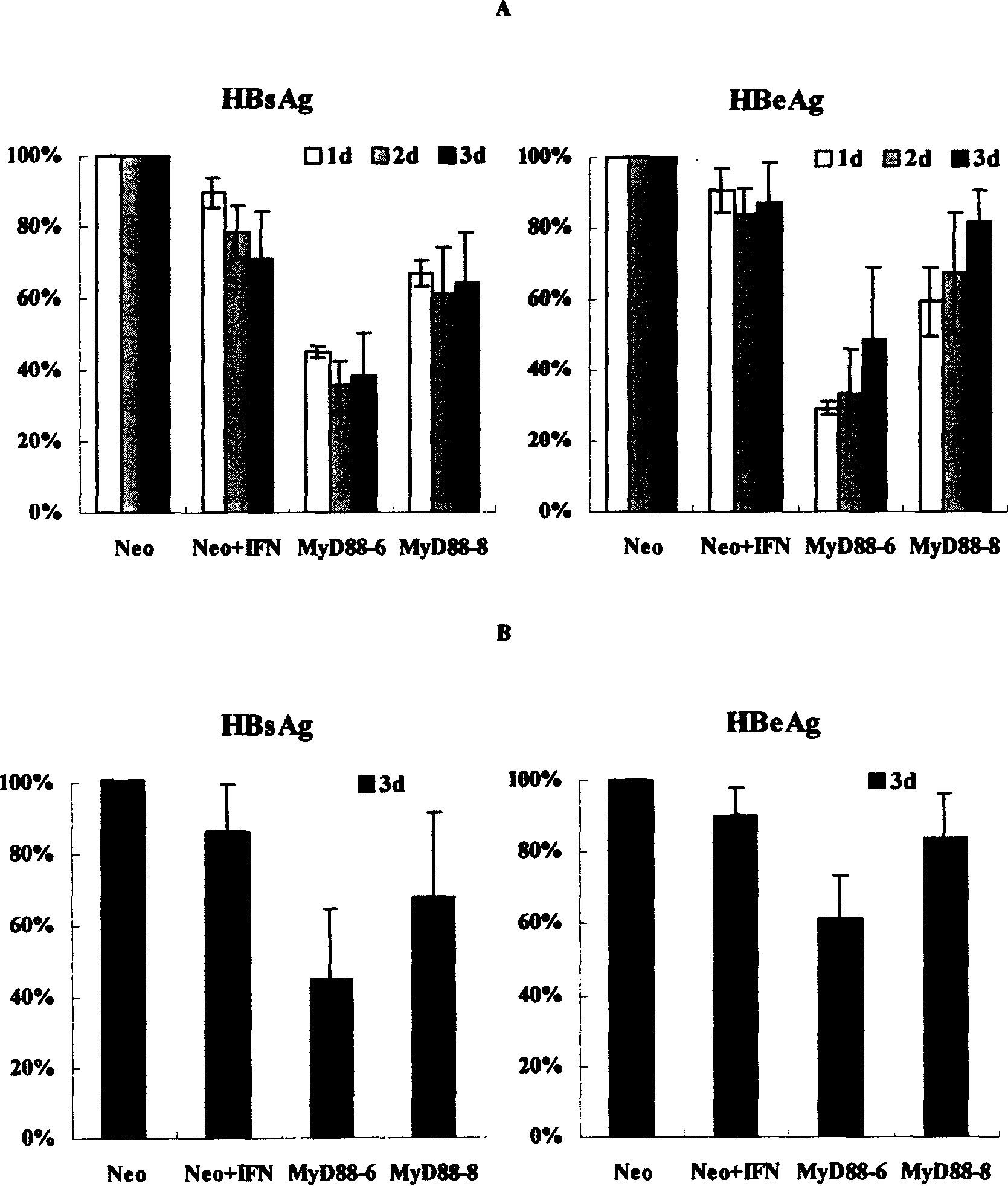
[0044] The influence of embodiment 2MyD88 on HBV protein synthesis
[0045] Inoculate HepG2 cells, MyD88 stable expression cell lines and Neo cell lines in 6-well plates, 3×10 per well 5 After culturing overnight, pHBV3.8 (3 μg) and pcDNA-CAT (0.5 μg) were transiently transfected into the above cells by calcium phosphate precipitation method. After 16 hours, fresh medium was replaced, and 1000 IU / ml recombinant human IFN-α (R&D Company) was added to the medium of cells in the IFN treatment group. After continuing to culture for 1 day, 2 days, and 3 days, the cell culture supernatants were collected respectively, and the cells were lysed after three days of culture. The levels of HBsAg and HBeAg were detected by CAT ELISA kit (Roche Company) to detect the CAT activity in the transfected cells to balance the transfection efficiency. The experiment was repeated three times, with multiple wells each time.
[0046] The results confirmed that compared with the Neo cell line trans...
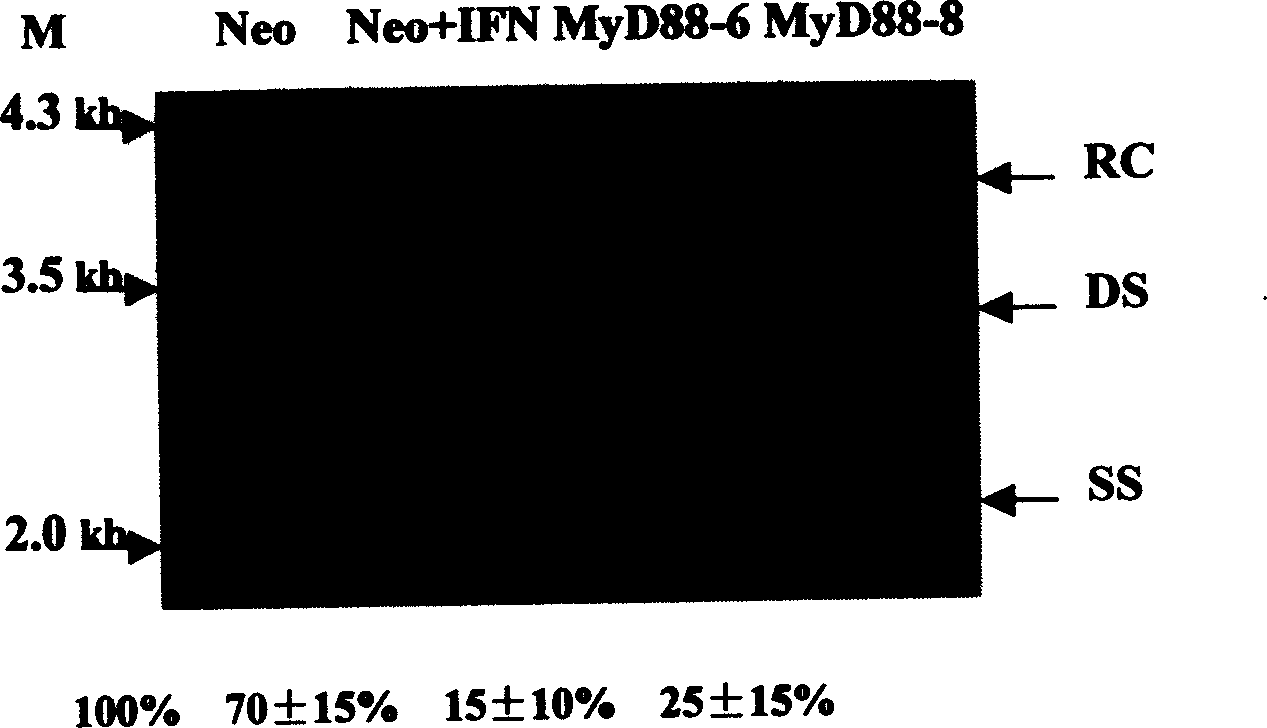
Embodiment 3
[0047] Example 3 Effect of MyD88 protein on HBV replication
[0048] HepG2 cells, MyD88 stable expression cell lines and Neo cells were divided into 1×10 6 cells / dish, inoculated on 60mm plate, transiently transfected the above cells with pHBV3.8 (10μg) and pcDNA-CAT (1.5μg) by calcium phosphate precipitation method, and added 1000IU / ml recombinant Human IFN-alpha. After 48 hours of transfection, the cells were washed once with PBS, and 650 μl of freshly prepared cell lysate (10 mmol / L Tris-HCl pH7.9, 1 mmol / L EDTA, 50 mmol / L NaCl, 1% NP40, 8% sucrose ), let stand at 37°C for 10min, repeatedly blow and blow to disperse the cell lysate, and centrifuge at 12000r / min for 2min at 4°C. Take 50 μl of the supernatant and use CAT ELISA to detect the CAT activity in the cells to balance the transfection efficiency, and extract the transfected cells according to the method of Lin Xu et al. HBV core particle DNA in the cytoplasm. According to the transfection efficiency, the loading ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com