Method for preparing procaine
A technology of procaine and Raney nickel, which is applied in the field of preparation of ester anesthetics, can solve the problems of expensive Pd/C catalyst, increased requirements for dangerous equipment, and limited application, etc., and achieves production cost reduction, The effect of short process route and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
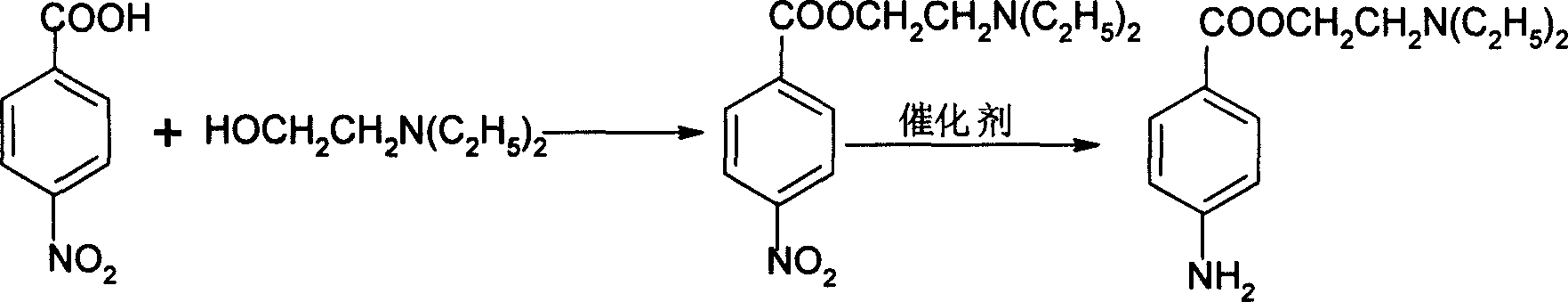
[0015] Weigh 20g of nickel-aluminum alloy, and add it into 160mL NaOH solution with a temperature of 60°C and a concentration of 19% in batches within 1.2h. Set aside, pour off the solution, wash twice with water at 75°C, and then wash with water at room temperature until the pH value is 8.5 to obtain a Raney nickel catalyst and store it in absolute ethanol.
[0016] In the 500ml three-necked flask that stirrer, thermometer, water trap are equipped with, add 46.4g (0.278mol) p-nitrobenzoic acid successively, 150ml xylene, 31g (0.264mol) N, N-diethylethanolamine, Heat to reflux to separate water. Stop the reaction after 19 hours, cool down to about 60°C, transfer to a separatory funnel, add 100ml of 5% NaOH solution preheated to about 60°C, let stand to separate layers, discard the water layer, and obtain 184ml of nitrocaine xylene solution , containing 0.204mol of nitrocaine, yield 73.4% (in terms of p-nitrobenzoic acid).
[0017] Add 120 mL of the obtained nitrocaine xylene...
Embodiment 2
[0019] Add 120 mL of the nitrocaine xylene solution obtained in Example 1, 130 mL of xylene and 4.2 g of Raney nickel catalyst wet weight into the reactor, seal it, maintain the temperature at 100 ° C, and the hydrogen pressure at 3.0 MPa. Other operations are the same as in Example 1 The measured hydrogenation reduction conversion rate was 93.1%, and 24.9 g of procaine was obtained as a pale yellow solid, with a yield of 79.2% and a purity of 97.8%.
Embodiment 3
[0021] Add 120 mL of the nitrocaine xylene solution obtained in Example 1, 130 mL of xylene, and 1.8 g of wet weight of Raney nickel catalyst into the reaction kettle, seal it, maintain the temperature at 130° C., and the hydrogen pressure at 2.0 MPa. Other operations are the same as in Example 1. , the hydrogenation reduction conversion rate was measured to be 90.3%, and 24.5 g of procaine was obtained as a pale yellow solid, with a yield of 77.8% and a purity of 97.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com