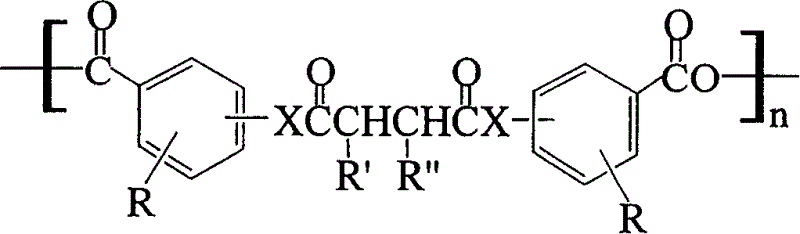Biodegradable fluorescent poly-anhydride and method for preparing same
A biodegradable polyanhydride technology, applied in the field of biodegradable fluorescent polyanhydride and its preparation, can solve the problems of insufficient degradation speed adjustment range and low mechanical properties, and achieve the effects of excellent mechanical properties, low price and excellent luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] p-Hydroxybenzoic acid (0.08mol) was dissolved in 70ml THF, pyridine (0.096mol) was added, succinyl chloride (0.088mol) was dissolved in 12ml THF, and slowly added dropwise to the p-hydroxybenzoic acid solution with a constant pressure funnel. After the mixed solution continued to react for 2 hours, the reaction mixture was poured into 500 ml of ice water, the pH value of the solution was adjusted to about 2 with hydrochloric acid, the resulting precipitate was filtered out, and vacuum-dried to obtain the product p-carboxyphenylsuccinic acid diester (bis- CPS). 10g bis-CPS was refluxed in 100ml acetic anhydride for 2h, acetic anhydride was distilled off under reduced pressure in a constant temperature water bath at 50°C, the residue was extracted with dry anhydrous ether, magnetically stirred overnight, the ether was decanted, and the solid product was placed in a P 2 O 5 The prepolymer was obtained by vacuum drying in a vacuum desiccator. Accurately weigh a certain am...
Embodiment 2
[0019] Salicylic acid (0.08mol) was dissolved in 70ml THF, pyridine (0.096mol) was added, succinyl chloride (0.088mol) was dissolved in 12ml THF, and slowly added dropwise to the salicylic acid solution with a constant pressure funnel. After continuing the reaction for 2 hours, the reaction mixture was poured into 500 ml of ice water, and the pH value of the solution was adjusted to about 2 with hydrochloric acid, and then the resulting precipitate was filtered out and dried in vacuo to obtain the product (yield 90%), the prepolymer and The polymer synthesis was similar to Example 1.
Embodiment 3
[0021] Syringic acid (0.08 mol) was dissolved in 70 ml of THF, pyridine (0.096 mol) was added, succinyl chloride (0.088 mol) was dissolved in 12 ml of THF, and slowly added dropwise to the syringic acid solution with a constant pressure funnel. After continuing the reaction for 2 hours, the reaction mixture was poured into 500 ml of ice water, and the pH value of the solution was adjusted to about 2 with hydrochloric acid, and then the resulting precipitate was filtered out and dried in vacuo to obtain the product (yield 84%), the prepolymer and The polymer synthesis was similar to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com