Preparation method of agricultural fungicide difenoconazole
A technology of difenoconazole and agricultural fungicides, which is applied in the field of agricultural fungicides, can solve the problems of affecting product quality and yield, difficulty in separation and purification, and difficulty in achieving quality and yield, thereby improving production efficiency and yield. rate increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
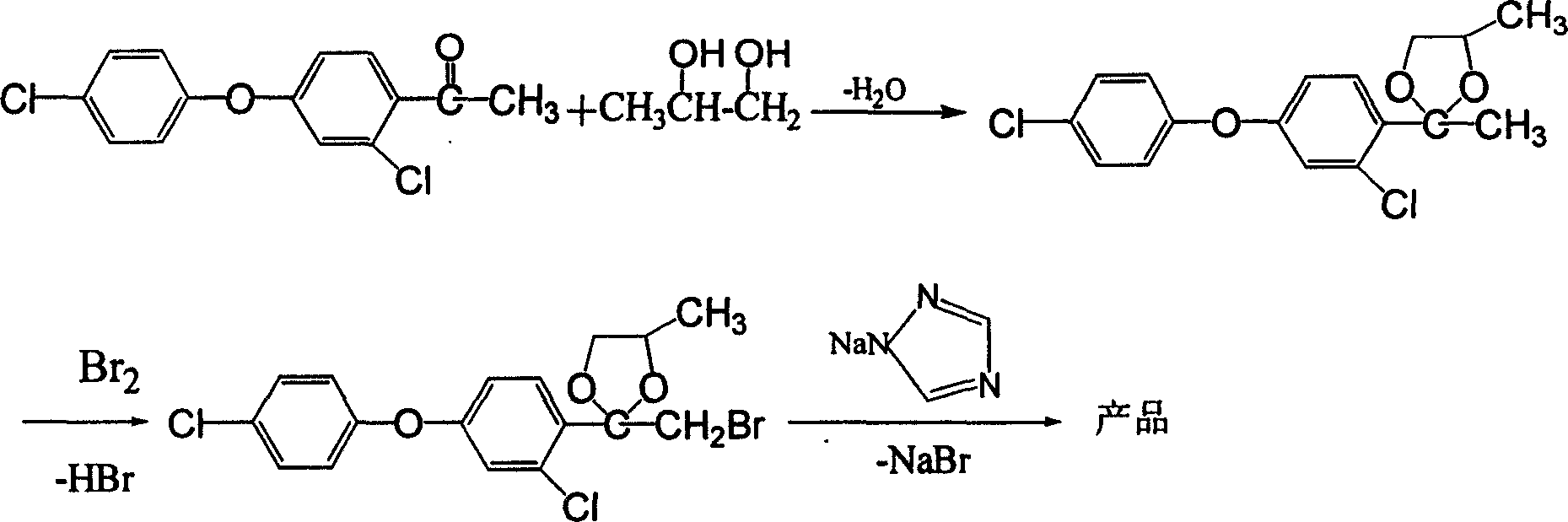
[0025] Synthesis of cis, trans-3-chloro-4-(2,4-dimethyl-1,3-dioxolan-2-yl)phenyl-4'-chlorophenyl ether:
[0026] 120g (99%, 0.42mol) 4-(4-chlorophenoxy)-2-chloroacetophenone, 48.5g (99%, 0.63mol) 1,2-propanediol, 4.5g (98%, 0.025mol) Put p-toluenesulfonic acid and 600mL toluene into the reaction bottle, stir and raise the temperature to reflux for dehydration, react for about 4 hours, GC analysis shows that acetophenone has been completely converted, finish the reaction and cool to room temperature, add 300mL / time water to wash three times, the organic layer is washed with Drying over anhydrous magnesium sulfate, 142.5 g of toluene-removed product under reduced pressure, GC showed that the content was ≥98%, and the yield was 98% (the ratio of cis-trans isomers was about 62.5 / 38.5).
[0027] Synthesis of cis, trans-3-chloro-4-(2-bromomethyl-4-methyl-1,3-dioxolan-2-yl)phenyl-4'-chlorophenyl ether:
[0028] 142.5g (98%, 0.412mol) of cyclic compounds, 5.5g (98%, 0.031mol) of p-to...
Embodiment 2
[0031] Embodiment 2 is basically the same as Embodiment 1, the difference is:
[0032] In the first step,
[0033] The solvent was changed to benzene;
[0034] The concentration mass ratio was changed to 5%;
[0035] In the third step
[0036] The polar solvent was changed to N,N-dimethylamide;
[0037] The concentration mass ratio was changed to 15%.
Embodiment 3
[0038] Embodiment 3 is basically the same as Embodiment 1, the difference is:
[0039] In the first step,
[0040] The solvent was changed to carbon tetrachloride;
[0041] Change the concentration mass ratio to 50%;
[0042] In the third step
[0043] The polar solvent was changed to N-methylpyrrolidone;
[0044] The concentration mass ratio was changed to 50%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com