Diagnostic test for determining the concentration of transient proteolytic activity in composite biological media
A proteolytic, active technology for use in diagnostics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
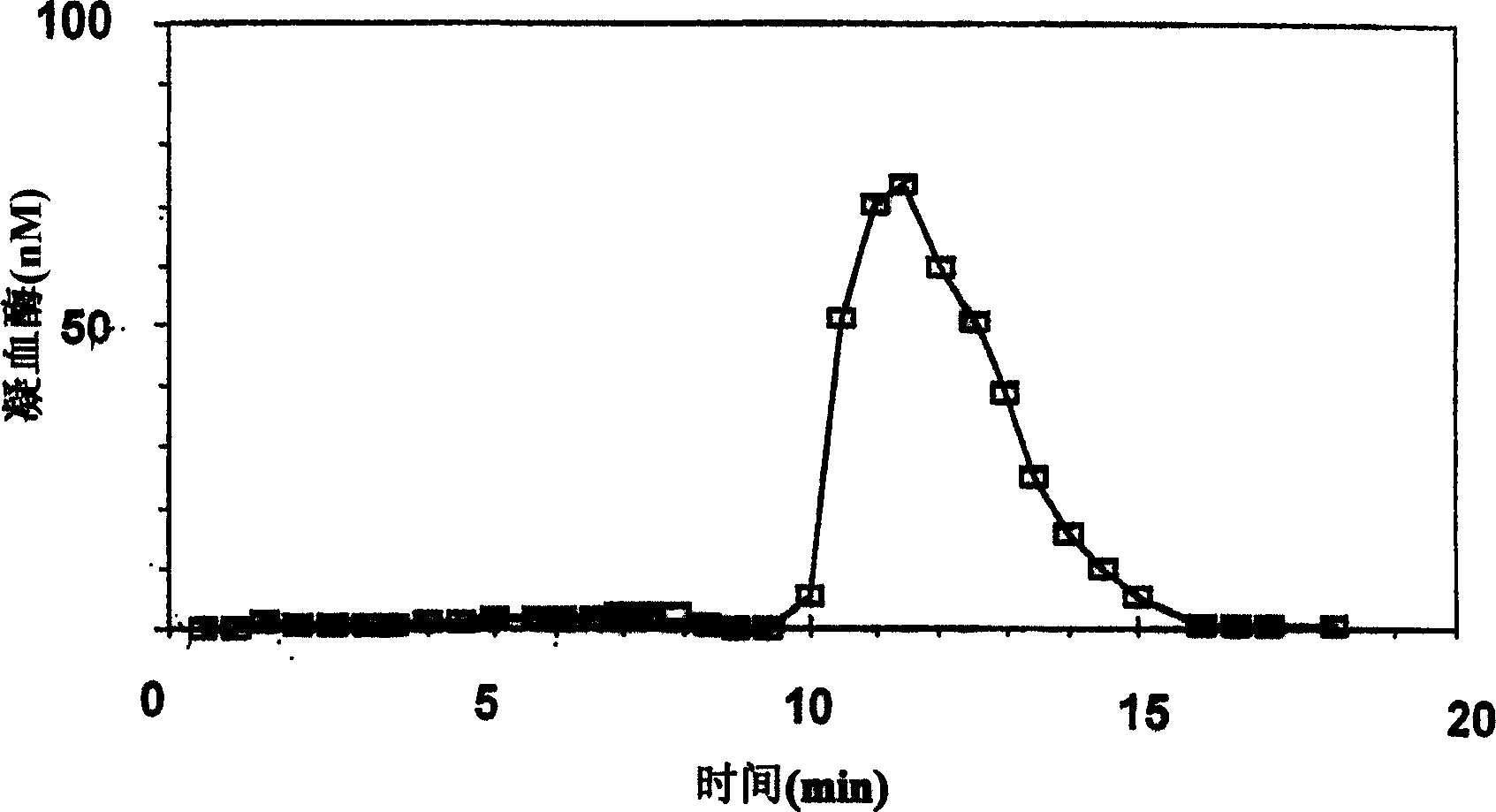
[0088] In order to practice the present invention, a typical description of the hemostatic and thrombosis system (HTS) will be given below, focusing on its most important enzyme, thrombin. It should be noted, however, that the present invention also relates to other enzymes and other physiological systems, such as other activated proteolytic enzymes (factors) of the coagulation system in blood and plasma, plasmin and other activators of the hemolytic fibrinolytic system in blood and plasma Components, complement factors activated in blood and plasma, pepsin in gastric juice, trypsin and chymotrypsin in duodenal juice, etc.
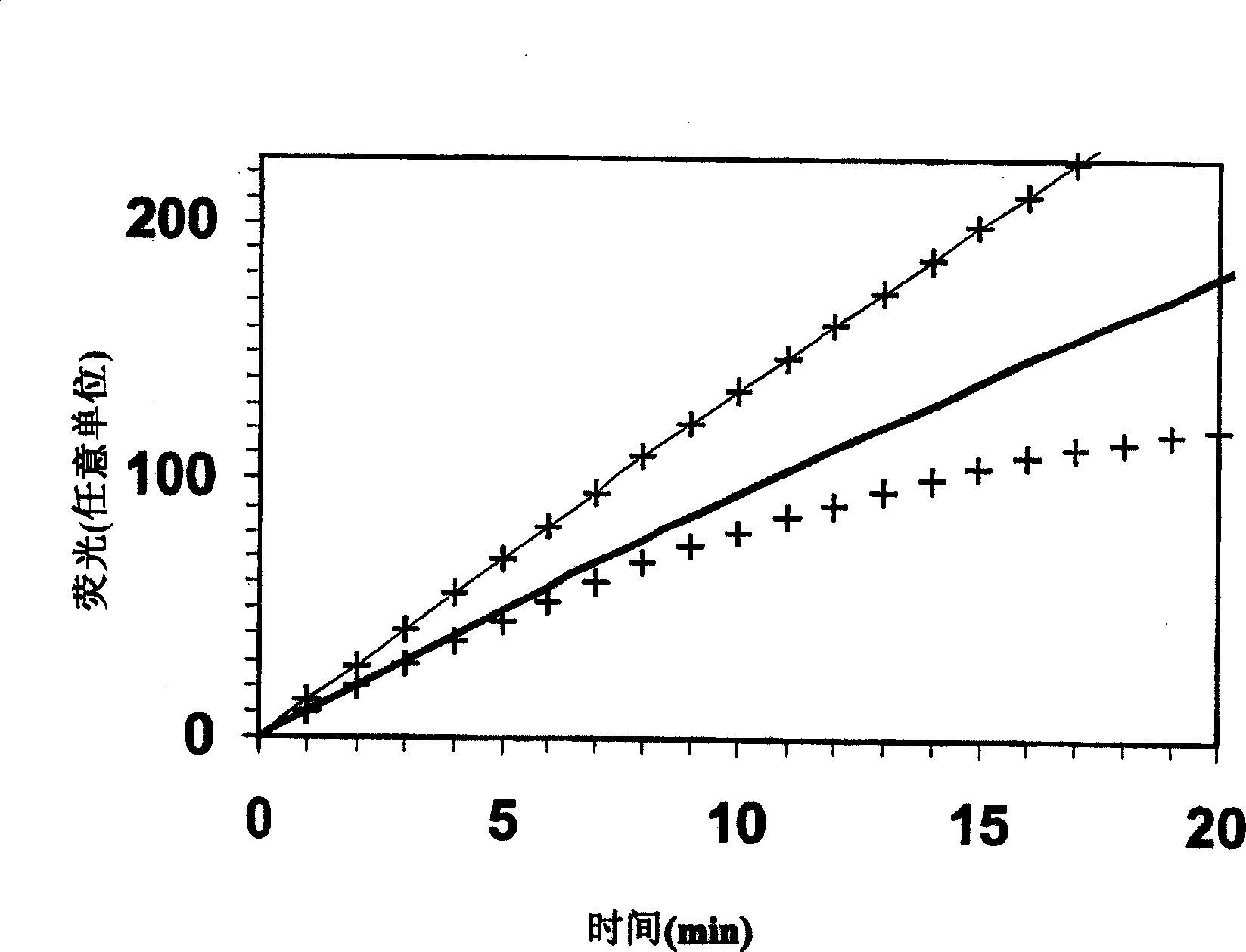
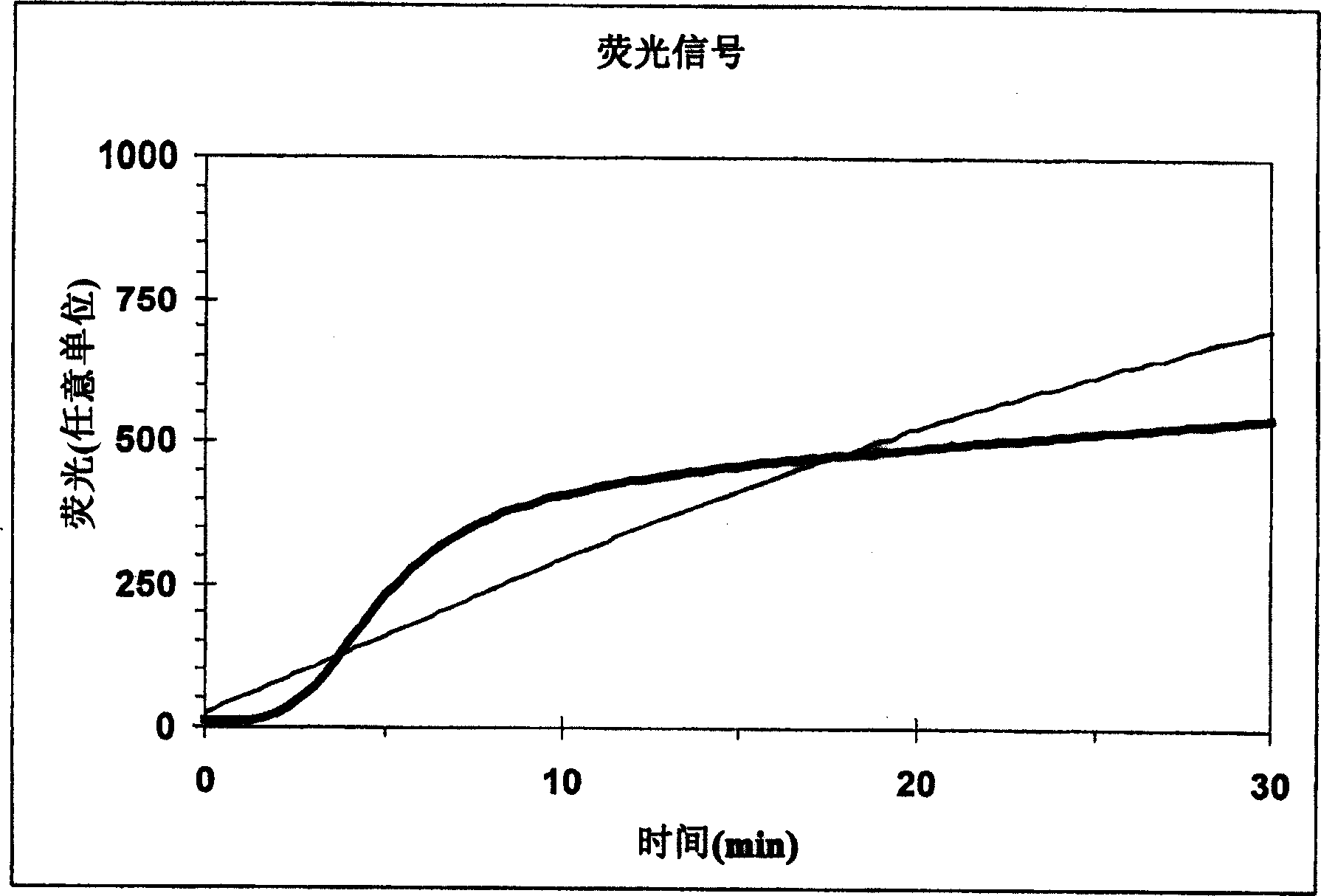
[0089] Typically, transient enzyme activity can be measured by adding a substrate that produces a signal upon transition, as described above. Where fluorogenic substrates are employed in the art, the method typically involves adding the fluorogenic substrate to blood (or another biological sample) in which thrombin production has been triggered (using meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com