Composition for treating or preventing hyperuricemia
A kind of technology of hyperuricemia and composition, applied in the field of composition for treating or preventing hyperuricemia, and can solve problems such as gout attack
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0029] 7-week-old SD female rats (n=5, 10 in total) were forcibly administered intragastrically with shark-derived chondroitin sulfate protein complex (SCP: manufactured by Maruha Co., Ltd.) or distilled water (control), at a rate of 1g amount, given for 14 days. After the administration, blood was collected from the ventral aorta, serumized, and GOT, GPT, total cholesterol, HDL-cholesterol, glucose, total bilirubin, total protein, albumin, urea nitrogen, creatinine, Ca, Na, Cl, alkaline phosphatase (ALP), uric acid, a total of 15 biochemical tests. As a result, the concentration of uric acid in the SCP group was significantly lower than that in the control group (p<0.01). The results are shown in Table 1 below.
[0030] control group
[0031] n.s.: no significant difference
experiment example 2
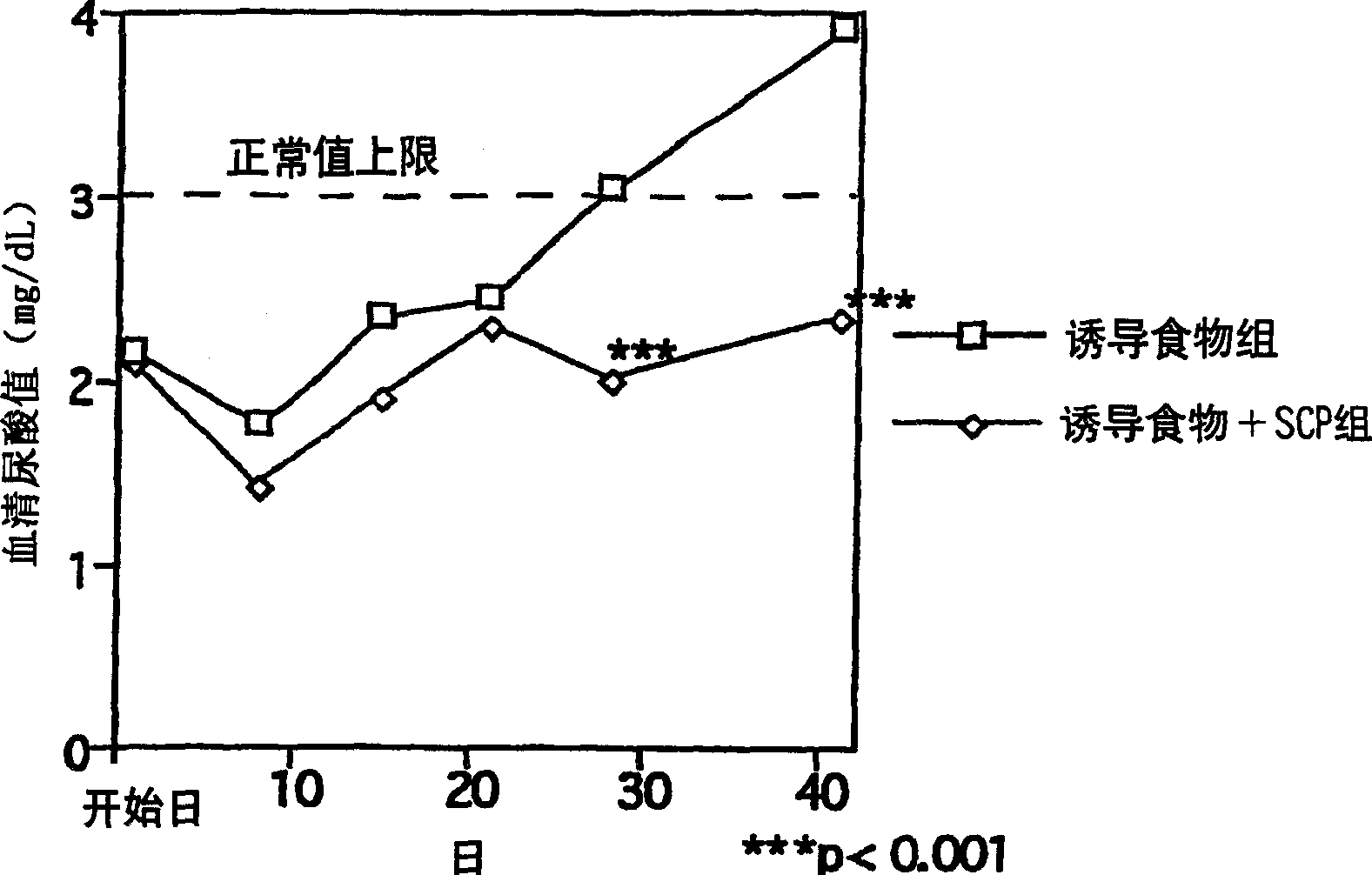
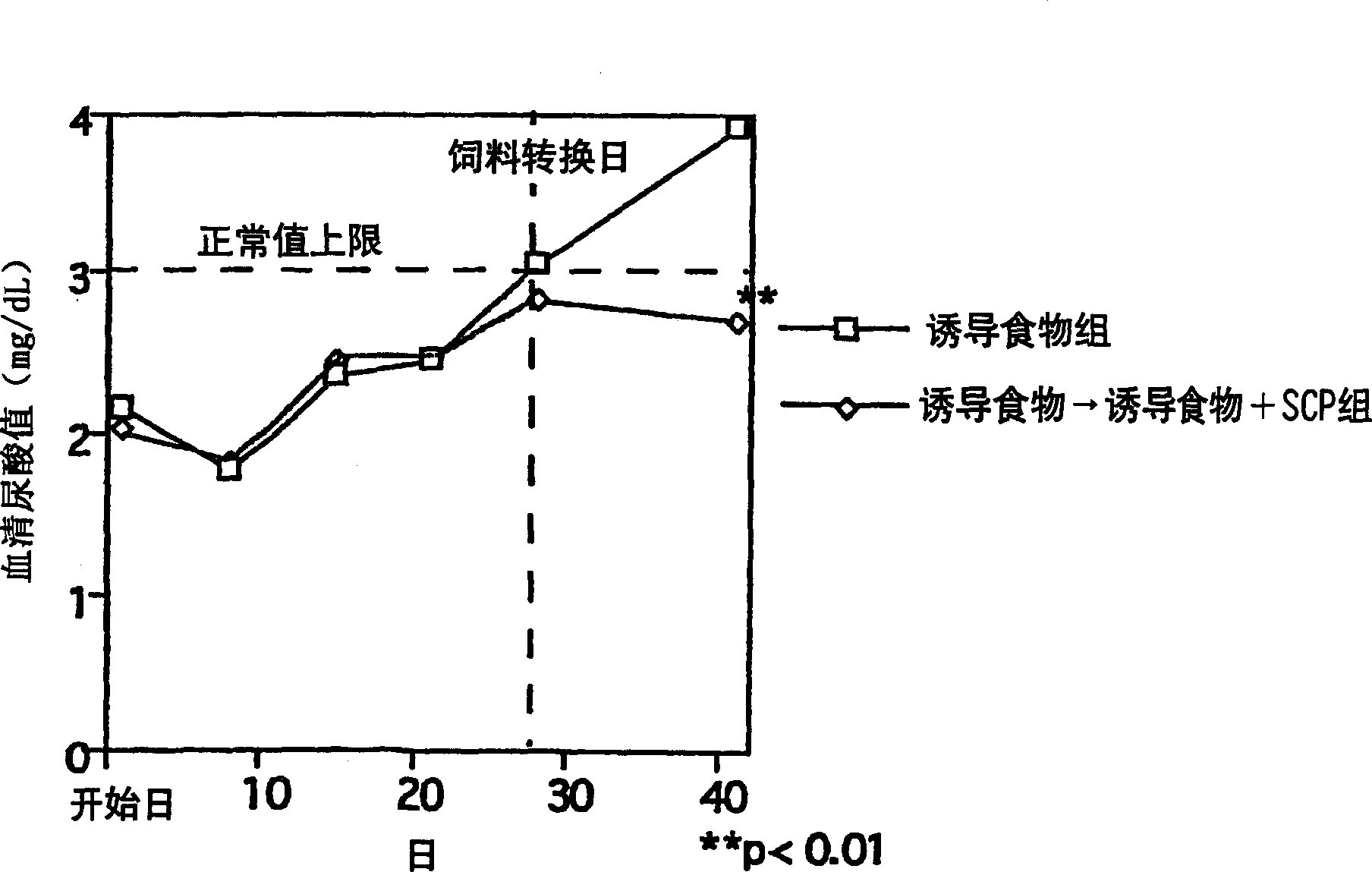
[0033]After the domestication and feeding, 10-week-old SD female rats (n=6, 18 in total) freely ingested the following feed: the Oriental compound feed produced by Oriental Yeast Co., Ltd. contained 2.5% by mass of the total amount of induced hyperuricemia oxonate potassium (potassium oxonate) feed (inducing food), or the feed (inducing food) further contains 1.5% by mass of the total amount of chondroitin sulfate protein complex (SCP: produced by Maruha Co., Ltd. ) feed (induction + SCP food) (10-15g / day), and track the change of serum uric acid value. The experimental group was set up as "induction food group" and "induction food + SCP group", and when there was a difference in serum uric acid value between the two groups, the "induction food → induction food + SCP group" was changed from induction food to induction + SCP food , a total of 3 groups. As a result, regarding the serum uric acid value, the "inducing food group" gradually increased, while the "inducing food + SC...
experiment example 3
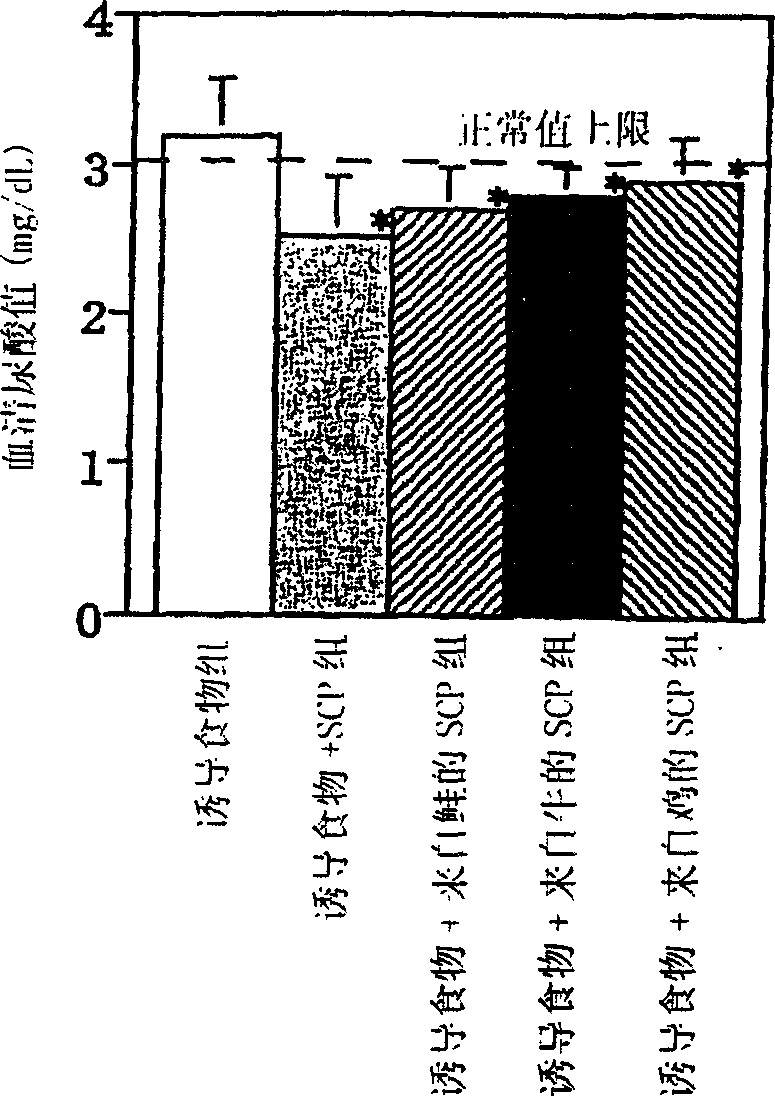
[0035] After the acclimatization and feeding, 4-week-old Wister male rats (n=6, 60 in total) were fed with the following feed: Oriental compound feed produced by Oriental Yeast Co., Ltd. containing 3% by mass of the total amount of induced hyperuricemia The feed (inducing food) of oxonic acid potassium salt, or the chondroitin sulfate protein complex (SCP, from salmon, SCP from cows, SCP from chickens: all were produced by Maruha Co., Ltd.) feed (10-17.5g / day), and the changes in serum uric acid value were tracked. As a result, the serum uric acid value of the four "induction+SCP" groups was significantly lower than that of the "induction food" group on the 28th day after ingesting the feed (p<0.05). The result is shown in Figure 3.
[0036] Judging from the results of the above experimental examples, the chondroitin sulfate protein complex has the effect of lowering the serum uric acid level.
[0037] The following are examples of the composition for treating or preventing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com