Injectable chondrocyte implant
A technology of chondrocytes and compositions, applied in bone implants, joint implants, joint implants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 - Chondrocyte Collection and Culture
[0089] By adding 2.5 ml of gentamicin (gentomycin) sulfate (concentration 70 micromol / liter), 4.0 milliliters of amphotericin (concentration 2.2 micromol / liter; trade name Fungizone®, an antifungal agent obtained from Squibb) , 15 milliliters of 1-ascorbic acid (300 micromol / liter), 100 milliliters of fetal calf serum (final concentration 20%), and the rest of the DMEM / F12 medium were mixed together to produce about 400 milliliters of growth medium, to prepare to transfer and / or process cartilage biopsies during culture and the growth medium used to culture chondrocytes (hereinafter "growth medium"). (The same growth medium is also used to transfer cartilage biopsies from the hospital to the laboratory, where the chondrocytes are extracted and propagated).
[0090] For autologous implants, cartilage biopsies are first collected by arthroscopic techniques, eg, from non-weight-bearing areas of the patient's joint, and tran...
Embodiment 2
[0096] Example 2 - Implantable Compositions
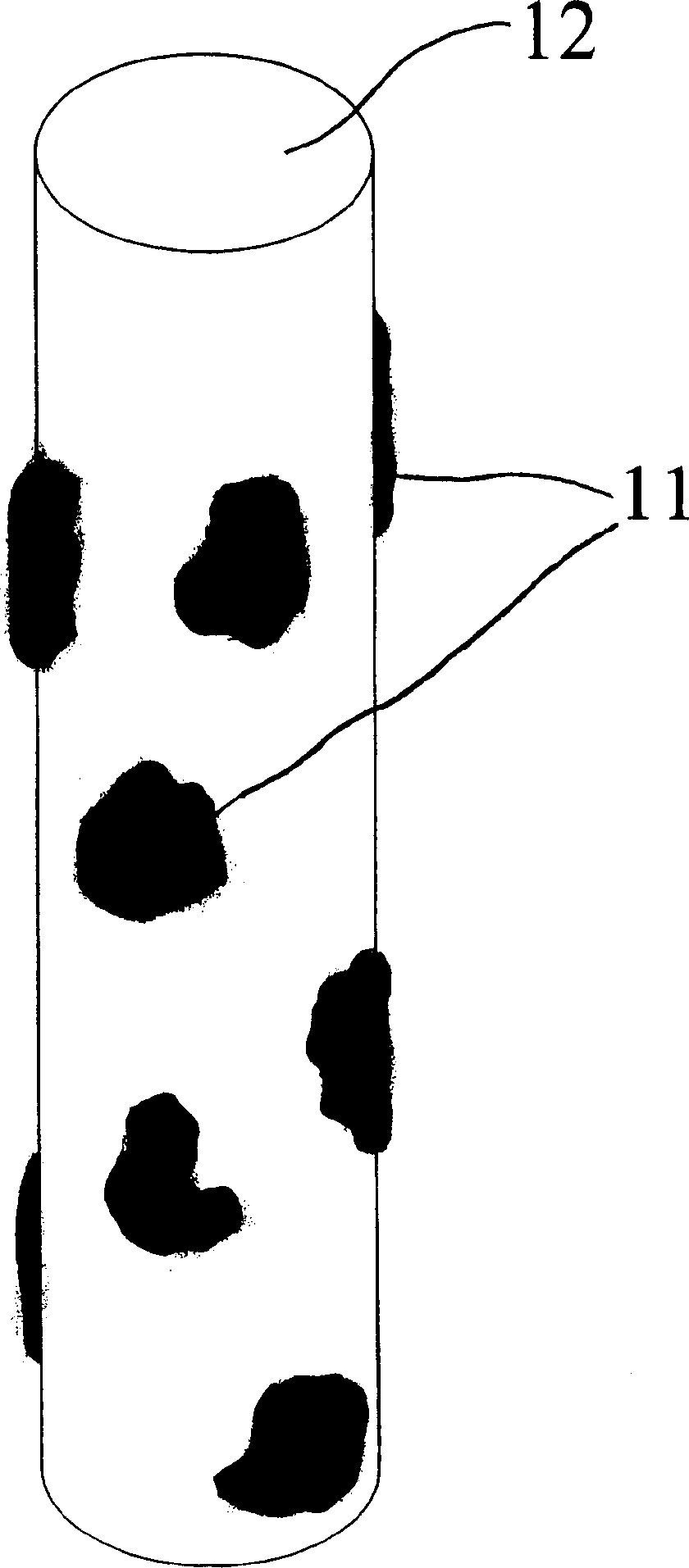
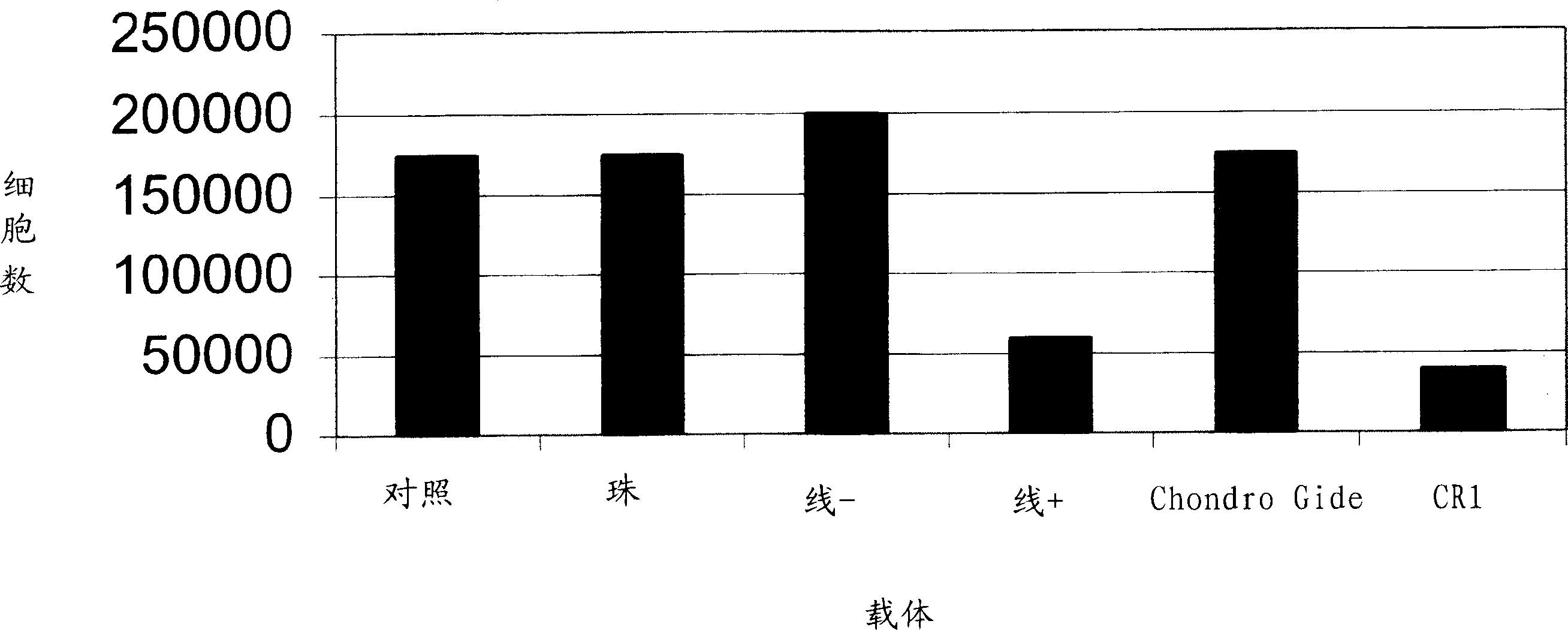
[0097] A carrier material of choice, such as a biocompatible, absorbable bead, mesh or thread, is mixed into transplantation medium in a sterile petri dish, the carrier material is "wetted" with the transplantation medium, and in one embodiment, The carrier material is exposed to the transplant medium for 1-10 hours or more. Chondrocytes are then added to the carrier material transplant medium mixture. Transplant medium can be 20% minimal essential medium with HAM F12 and 15 mM Hepes buffer and 10 to 20% autologous serum, all maintained at 37 °C in CO 2 in the incubator.
[0098] The chondrocytes are then contacted and grown on or in the carrier material for a period of time ranging from 1 hour to 1 week, and in one embodiment the chondrocytes are maintained at a temperature of about 37°C. Preferably, the chondrocytes are incubated overnight with the carrier material. In one embodiment, the chondrocytes and medium are gently ag...
Embodiment 3
[0102] Example 3 - Implantable Compositions
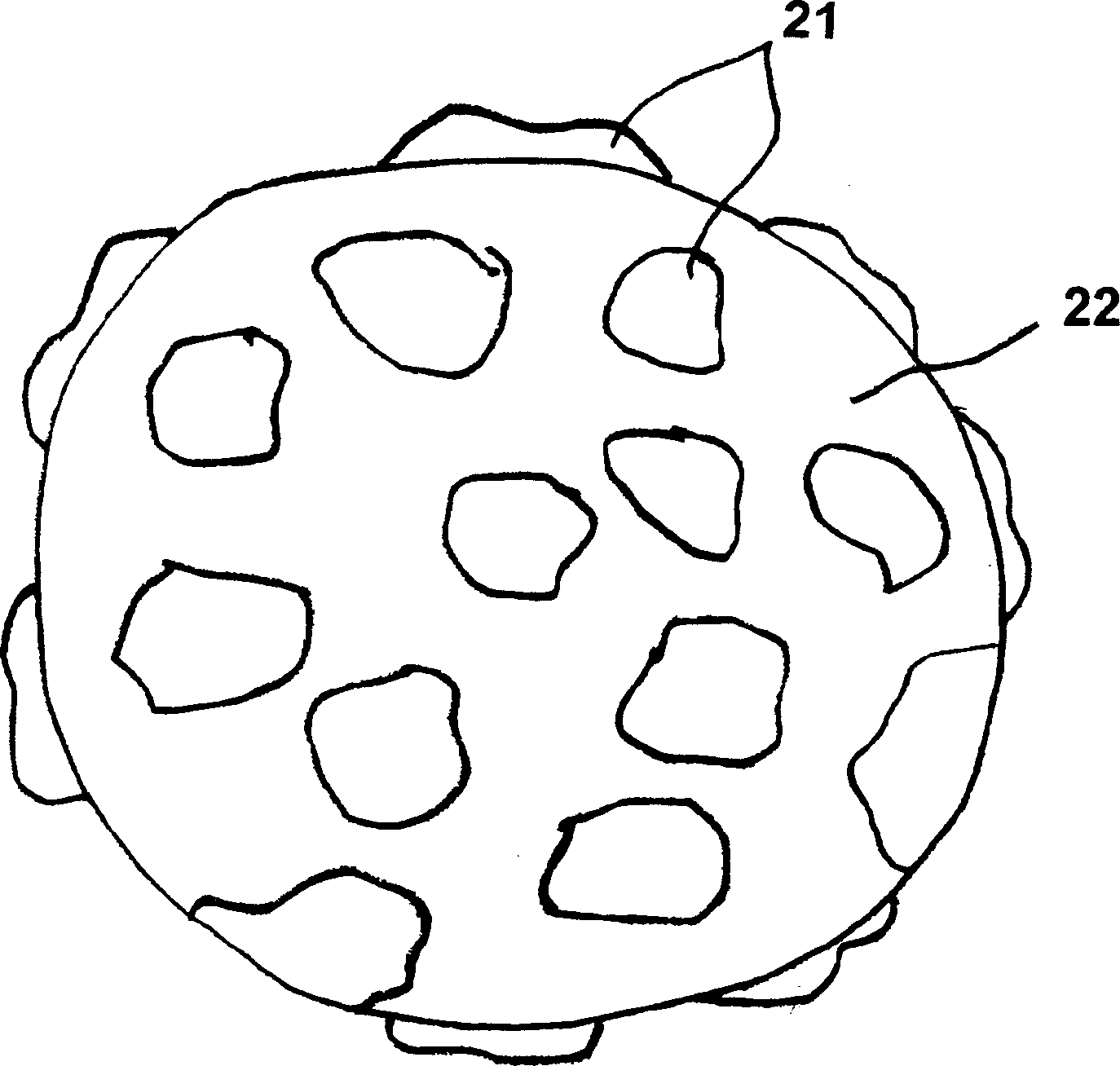
[0103] In another embodiment, chondrocytes suspended in culture medium can be added directly to the carrier material without "pre-wetting" the carrier material. In this case, the chondrocytes are then allowed to attach to and grow on or in the carrier material for a period of time ranging from about 1 hour to about 6 weeks. Preferably, the chondrocytes are incubated overnight with the carrier material. In one embodiment, the chondrocytes and medium are gently agitated so that the chondrocytes adhere to and grow on all sides of the carrier material.
[0104] In another embodiment, additional support material (the same or different than the original support material) is added during the culture to expand the cell culture. In another embodiment, the carrier material is first disrupted by using an enzyme such as trypsin. Additional support material having a surface area greater than the destroyed original support material is then ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap