Human telomerase active inhibitor protein and use thereof
A protein polypeptide and sequence technology, applied in the field of biotechnology and medicine, can solve the problems of limited protein and no protein found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1: Cloning of MCRS2 protein cDNA
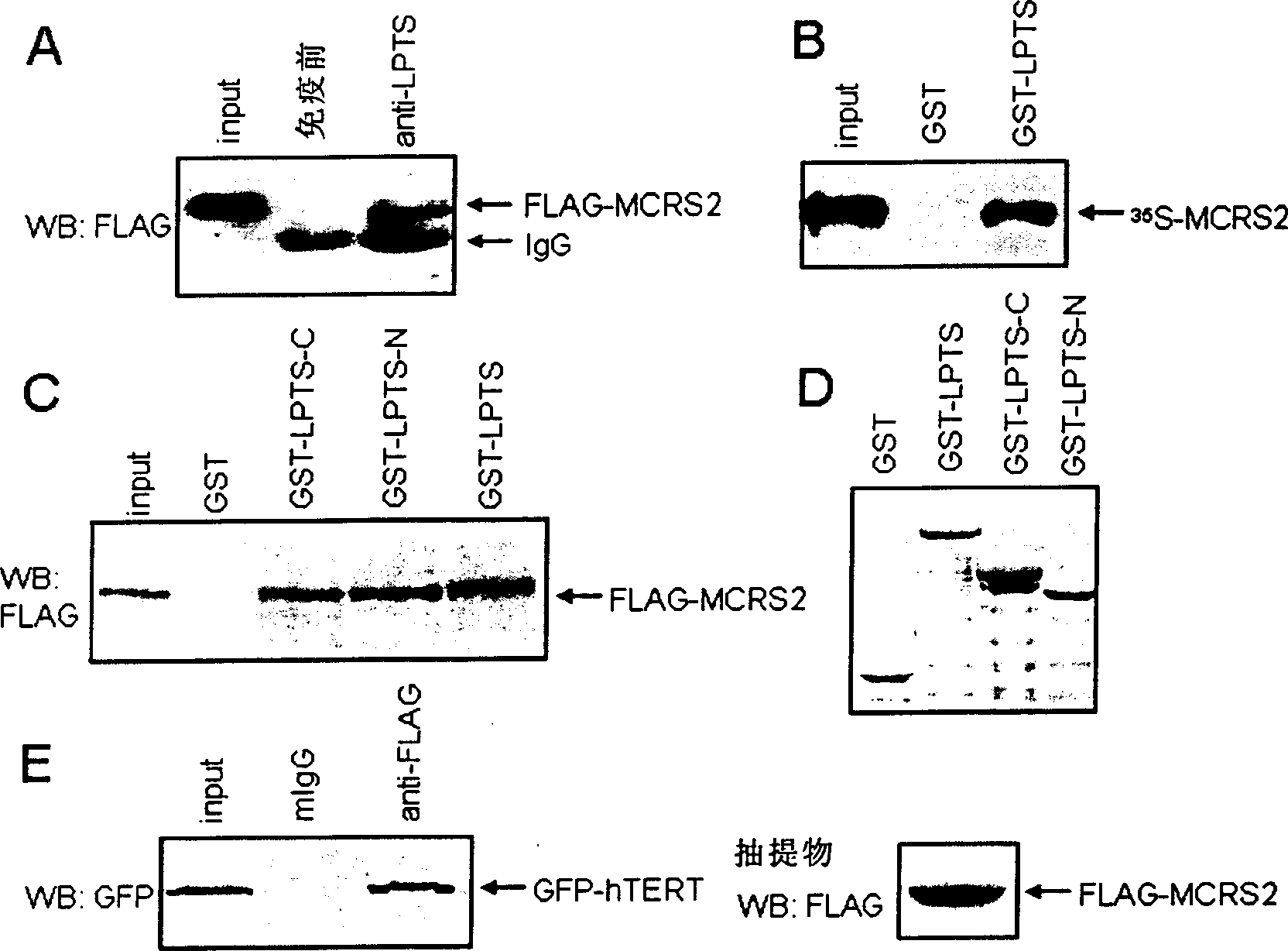
[0096] The interacting proteins of LPTS were screened by the conventional yeast two-hybrid technique, and the cDNA encoding LPTS was inserted into the pGilda vector (purchased from Clontech Company) for screening the human brain cDNA library (Clontech), according to the operating procedures provided by the company. The screened positive clones were verified and sequenced, and the sequences were compared with the GenBank database.
[0097] According to sequence information, design primer, carry out polymerase chain reaction (PCR), PCR reaction primer is: P1 (SEQ ID NO: 3), P2 (SEQ ID NO: 4), with the cDNA library plasmid of liver (GIBCO BRL company product ) as a template, PCR amplification to obtain MCRS2 gene cDNA. The PCR reaction conditions were 25 μl volume, pre-denaturation at 94°C for 4 minutes, cycle reaction at 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 2 minutes, for a total of 35 cycles. PCR products ...
Embodiment 2
[0099] Embodiment 2, recombinant expression and purification of MCRS2 protein
[0100] (a) Recombinant expression of MCRS2 protein
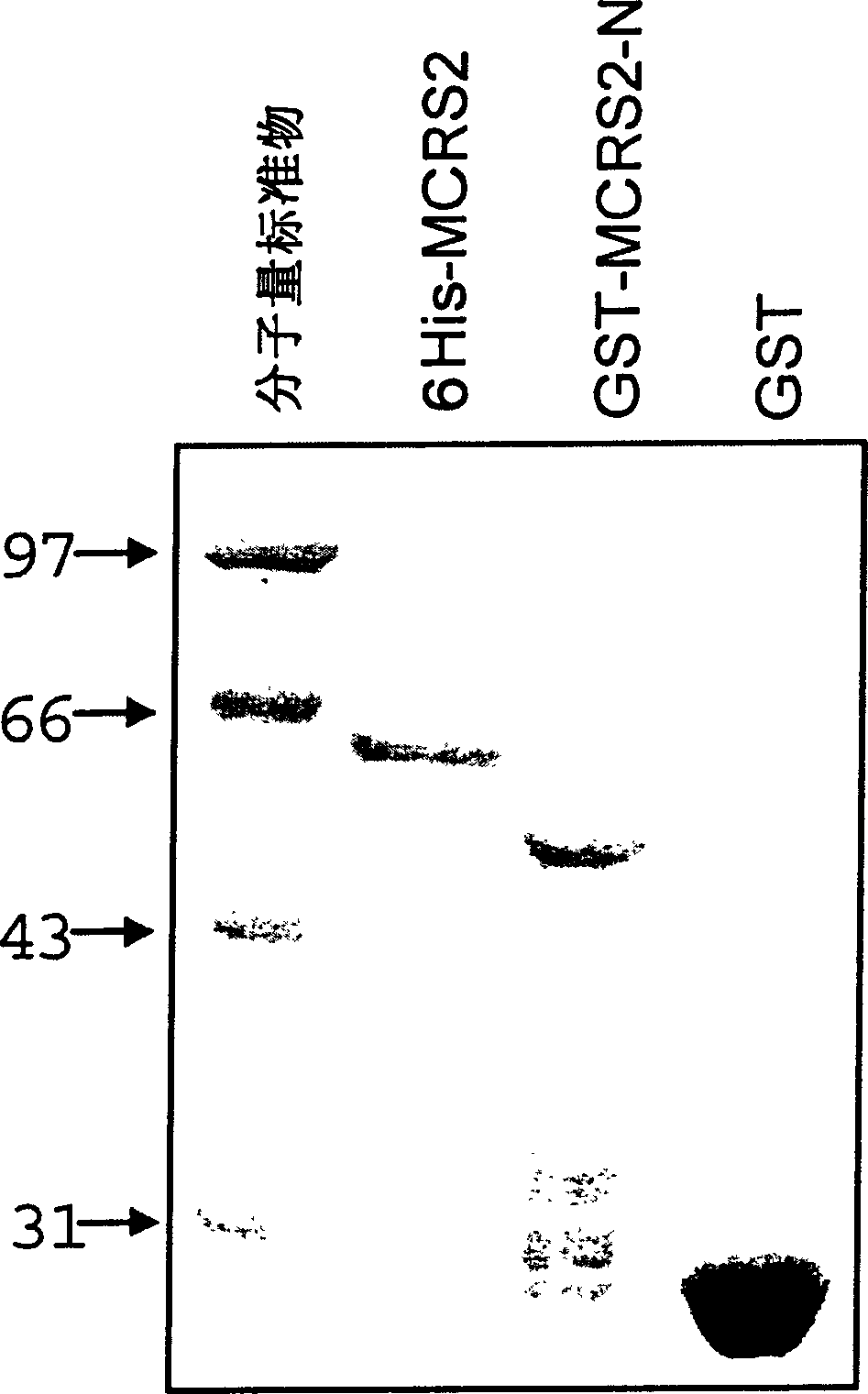
[0101] The MCRS2 nucleotide sequence obtained in Example 1 was digested with EcoRI and XhoI and loaded into the same digested 6×His-fusion expression vector pET-30 ((Novagen Company) to obtain the expression vector 6×His-MCRS2 .
[0102] The cDNA fragment (corresponding to 173-658 in SEQ ID NO: 1) containing MCRS2 N-terminal 162 amino acids was loaded into the GST-fusion expression vector pGEX-4T1 (Amersham Company) through EcoRI and XhoI restriction sites, and the obtained The plasmid was named GST-MCRS2-N.
[0103] Then the constructed plasmid was transformed into Escherichia coli BL21-DE3 to obtain MCRS2 / BL21-DE3 and MCRS2-N / BL21-DE3 transformed strains. After the obtained positive transformed strains were amplified, the plasmids were extracted and identified with corresponding restriction endonucleases, and confirmed by DNA sequencing.
...
Embodiment 3
[0110] Embodiment 3, the preparation of anti-MCRS2 protein antibody
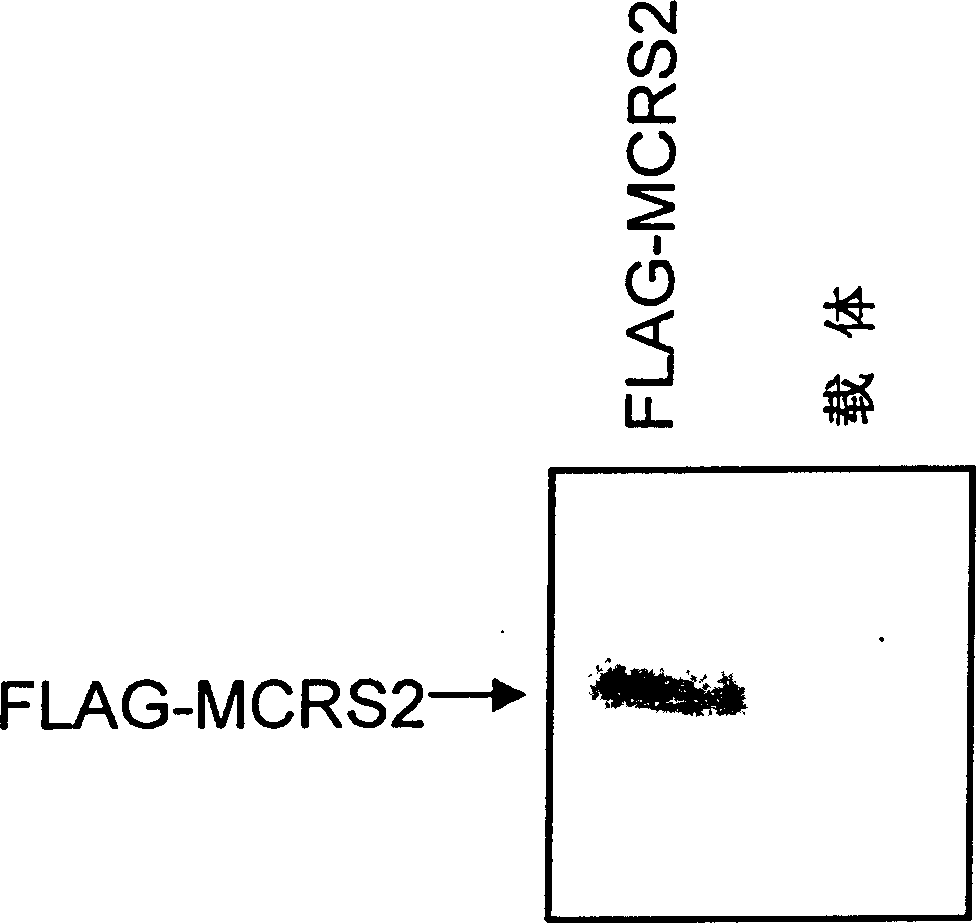
[0111] Take 300 μg of purified 6×His-MCRS2 fusion protein, dissolve it in 0.5 mL PBS, add an equal volume of Freund’s complete adjuvant, mix well, and inject into the back of 2.0 kg male New Zealand white rabbits intradermally at multiple points. On the third day, the same amount of protein was mixed thoroughly with Freund's complete adjuvant again, and then intradermally injected in multiple points on the back. On the 28th day, the same amount of protein was mixed with Freund's incomplete adjuvant, and injected intradermally on the back to boost the immunization. After one week, the carotid artery was bled to collect blood, placed at 37°C for 3 hours, and placed at 4°C overnight to completely precipitate the serum, collected by centrifugation, aliquoted, and stored at -70°C. The specific detection method of the antibody is as follows: 1.5 μg of FLAG-MCRS2 plasmid was mixed with 10 μl of Lipofectamine (prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com