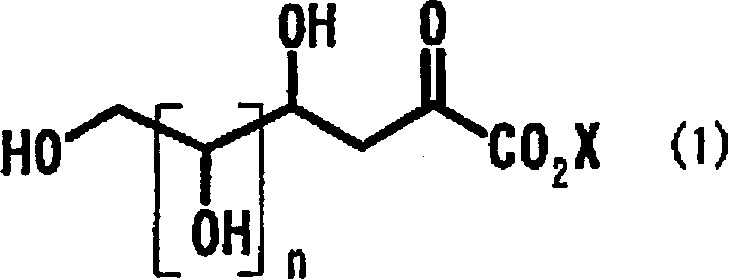
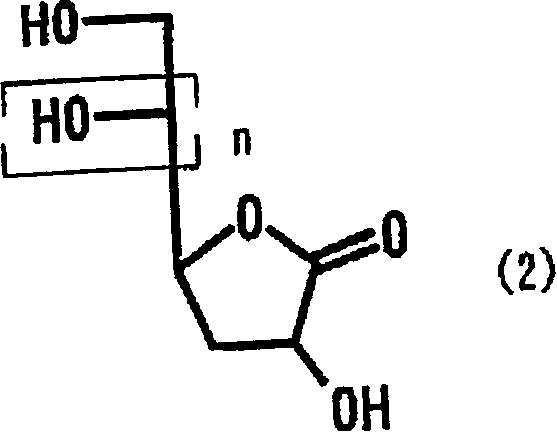
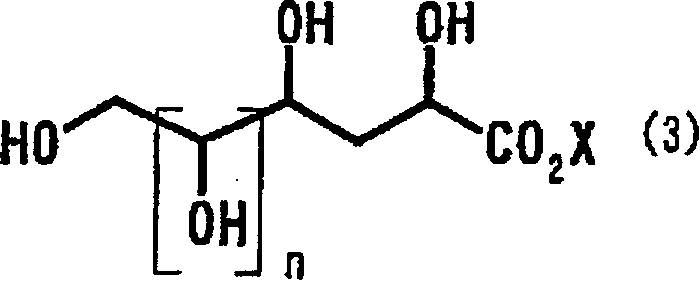
Process for producing 2-deoxyaldose compound
A technology of alkaline earth metals and general formulas, which is applied in the preparation of sugar derivatives, chemical instruments and methods, monosaccharides, etc., can solve the problems of unsatisfactory safety and economic efficiency, unsatisfactory, high cost, etc., to achieve Excellent volumetric efficiency, easy recycling, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] [Example 1] Contact hydrogenation of 2-keto-3-deoxy-D-gluconic acid
[0091] Add 50 mg of 10% palladium carbon (48% aqueous product) to 5.0 g of a 10% aqueous solution of 2-keto-3-deoxy-D-gluconic acid (hereinafter referred to as KDG), and heat to an internal temperature of 48°C. After reacting under hydrogen flow for 9 hours, the reaction solution was analyzed by HPLC, and the reaction yield of metasaccharic acid lactone was 54%, and the reaction yield of metasaccharic acid was 26%.
[0092] HPLC analysis conditions: Shodex Asahipack NH2-P50 (manufactured by Showa Denko), 50 mM sodium phosphate aqueous solution, flow rate 1 ml / min, UV210 nm detection.
Embodiment 2
[0093] [Example 2] The contact hydrogenation of KDG
[0094] The reaction was carried out in the same manner as in Example 1 except that 45 µl of sulfuric acid was added. The reaction solution was analyzed by HPLC, and the reaction yield was 92% of the mixture of metasaccharic acid lactone and metasaccharic acid.
Embodiment 3
[0095] [Example 3] The contact hydrogenation of KDG
[0096] The reaction was carried out in the same manner as in Example 1 except that 107 µl of sulfuric acid was added. The reaction solution was analyzed by HPLC, and the reaction yield was 96% of the mixture of metasaccharic acid lactone and metasaccharic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com