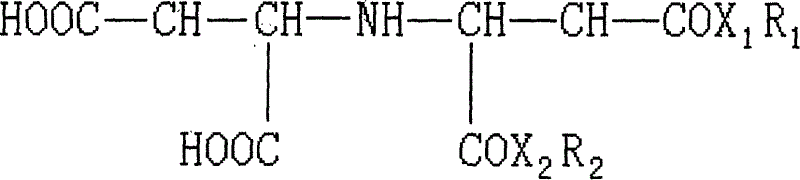
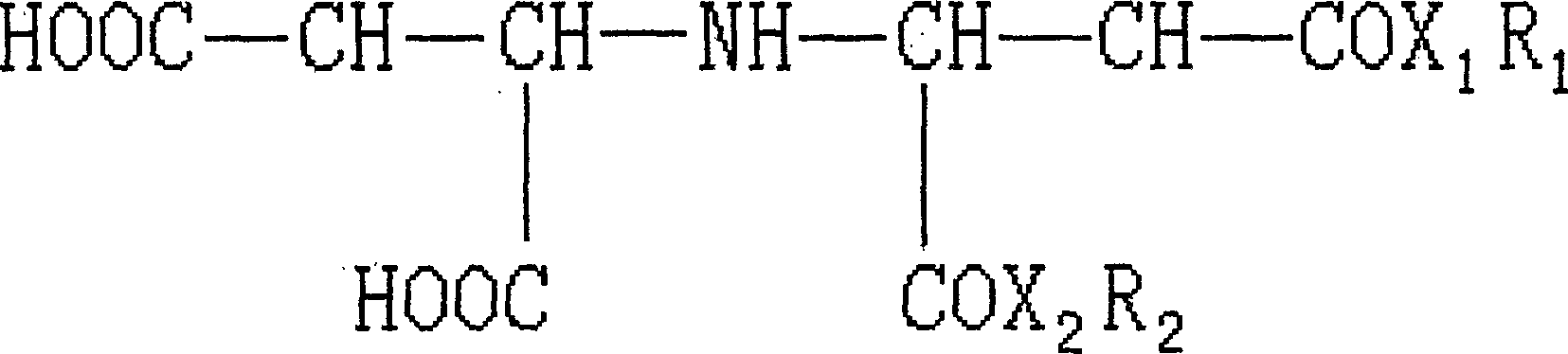
Chelated surfacant
A surfactant and chelating technology, applied in the field of surfactants, can solve the problems of EDTA degradability and complicated production process, and achieve the effect of strong surface activity and chelating ability, short process flow and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] 1. Synthesis of monolauryl iminodisuccinate
[0014] A whole set of Soxhlet extraction device is used as the reaction device. The flask was filled with 24.9 g (0.1 mol) of dry iminodisuccinic acid, 18.6 g (0.1 mol) of lauryl alcohol, 0.5 ml of sulfuric acid and 600 ml of solvent dimethylformamide. 100 grams of anhydrous magnesium sulfate are packed in a filter paper bucket in the extractor to absorb the water taken out in the reaction system. The reaction was stirred and heated to reflux for 15 hours, then stopped. The mixture in the flask was distilled under reduced pressure to recover most of the solvent, about 480 ml of dimethylformamide, the residue was cooled, poured into 1 liter of cold water to form a precipitate, stirred and washed the precipitate, filtered to collect the precipitate, the precipitate Pour it into 1 liter of 0.01 mole sodium bicarbonate solution, stir and wash, filter, wash the precipitate with water, and dry the precipitate under vacuum at 40-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com