Human blood high density lipoprotein and its preparation method and use
A technology of high-density lipoprotein and manufacturing method, applied in the field of biochemistry, can solve the problems of underutilization and waste, and achieve the effect of significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
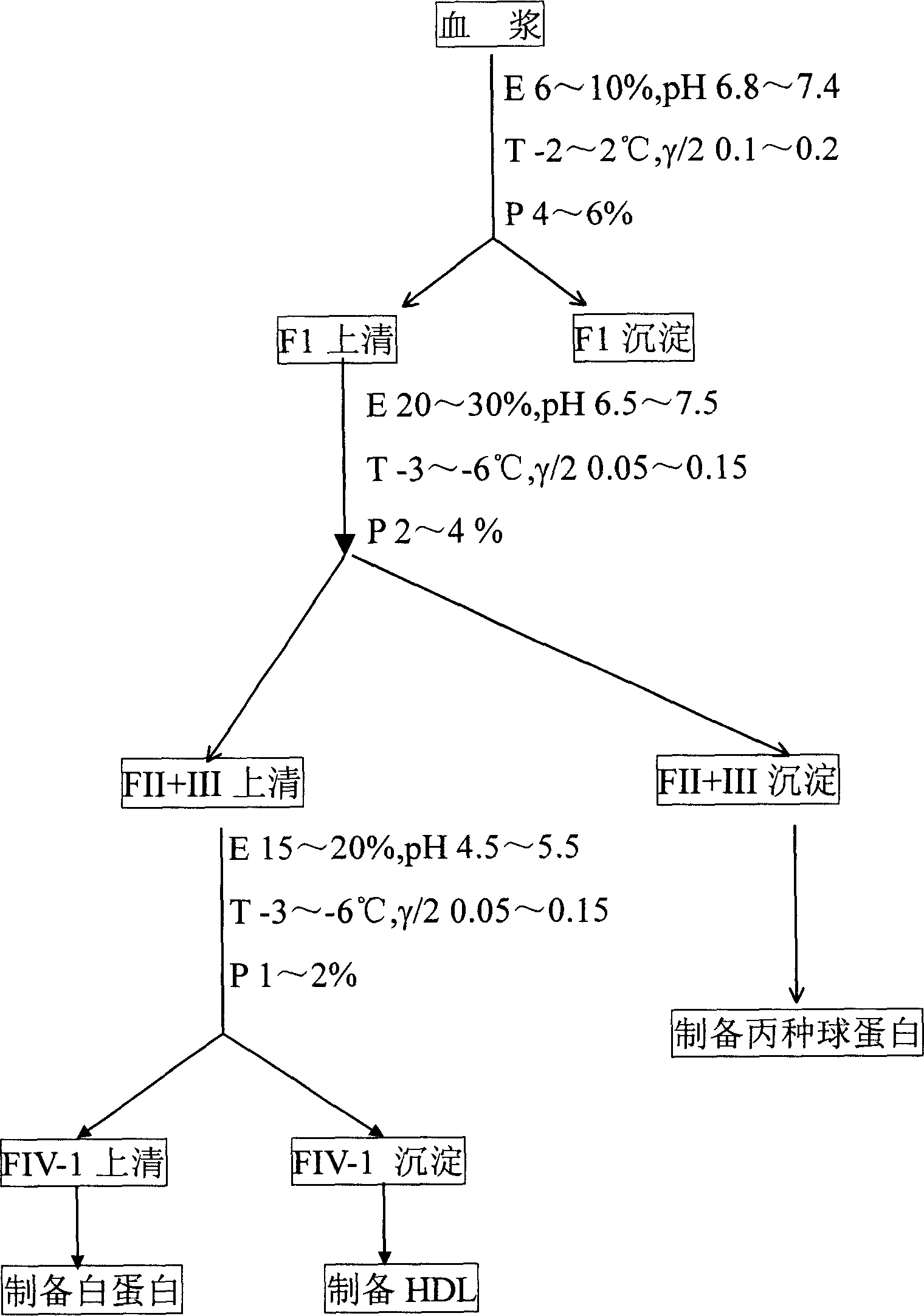
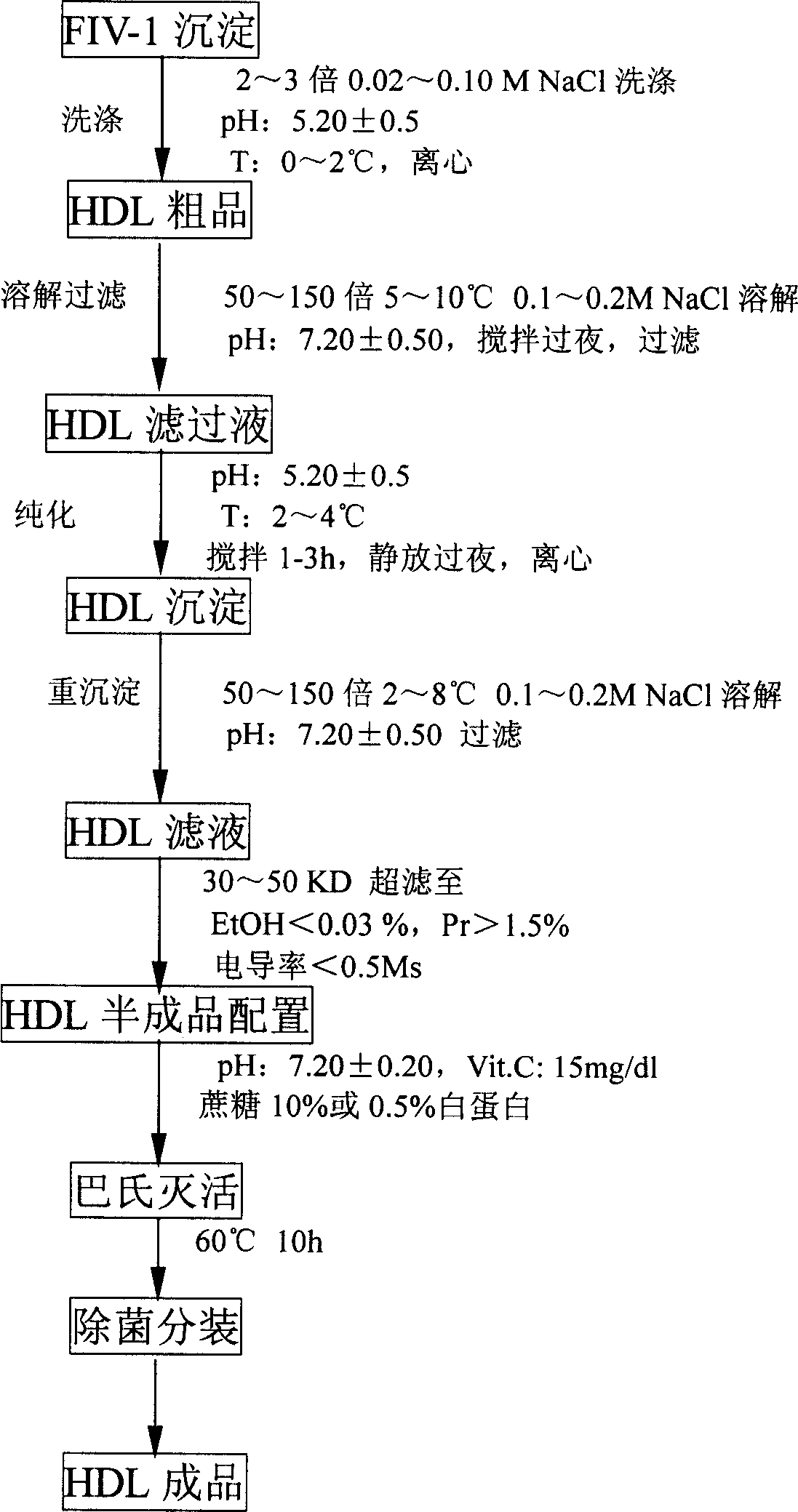
[0067] Take 1000L of raw plasma, add 190.5L of 50% ethanol, adjust the pH value to 7.1, protein concentration to 5.6%, and ionic strength to 0.15, stir at -2°C for 2 hours, let stand for 8 hours, use a 142-type continuous centrifuge, Centrifuge at 14000r / min to obtain 1190 L of supernatant and 12Kg of precipitate;
[0068]Add 5000 mL of HAC / NaAC mixed solution with a pH of 4.0 dropwise to the supernatant to make the pH of the mixed solution 6.90, add 263 L of 95% ethanol to make the protein concentration 3.4%, and the ionic strength 0.09, stir at -4°C for 2 hours, and statically Set aside for 8 hours, use a 142-type continuous centrifuge, and centrifuge at 14000r / min to obtain a supernatant of 1450L and a precipitate of 40Kg;
[0069] Add 682 L of water for injection to the supernatant, dropwise add 14.8 L of HAC / NaAC mixed solution with a pH of 4.0, so that the pH is 4.9, the protein concentration is 1.7%, and the ionic strength is 0.15. Place for 2 hours, use a 142-type con...
Embodiment 2
[0077] Take 1000L of raw plasma, add 190.5L of 50% ethanol, adjust the pH value to 7.3, protein concentration to 5.2%, and ionic strength to 0.18. Stir at -2°C for 2 hours, let stand for 8 hours, and use a 142-type continuous centrifuge. Centrifuge at 14000r / min to get supernatant 1100L and precipitate 12Kg;
[0078] Add 5000 mL of HAC / NaAC mixed solution with a pH of 4.0 dropwise to the supernatant to make the pH of the mixed solution 6.90, add 243 L of 95% ethanol to make the protein concentration 4.0%, and the ionic strength 0.15, stir at -6°C for 2 hours, and statically Set aside for 8 hours, use a 142-type continuous centrifuge, and centrifuge at 14000r / min to obtain a supernatant of 1449L and a precipitate of 39Kg;
[0079] Add 682 L of water for injection to the supernatant, dropwise add 14.8 L of HAC / NaAC mixed solution with a pH of 4.0, so that the pH is 5.0, the protein concentration is 2.0%, and the ionic strength is 0.09. Place for 2 hours, use a 142-type continuo...
Embodiment 3
[0087] Take 1000L of raw plasma, add 190.5L of 50% ethanol, adjust the pH value to 6.8, protein concentration to 4.0%, and ionic strength to 0.10, stir at -2°C for 2 hours, let stand for 6 hours, use a 142-type continuous centrifuge, Centrifuge at 14000r / min to obtain 1190L of supernatant and 13Kg of precipitate;
[0088] Add dropwise 5000 mL of HAC / NaAC mixed solution with a pH of 4.0 to the supernatant to make the pH of the mixed solution 6.90, add 263 L of 95% ethanol to make the protein concentration 2.0%, ionic strength 0.05, stir at -6°C for 2 hours, statically Set aside for 6 hours, use a 142-type continuous centrifuge, and centrifuge at 14000r / min to obtain a supernatant of 1450L and a precipitate of 38Kg;
[0089] Add 658 L of water for injection to the supernatant, dropwise add 14.3 L of HAC / NaAC mixed solution with a pH of 4.0, so that the pH is 4.5, the protein concentration is 1.0%, and the ionic strength is 0.05. Stir at -3°C for 2 hours, and statically Place fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com