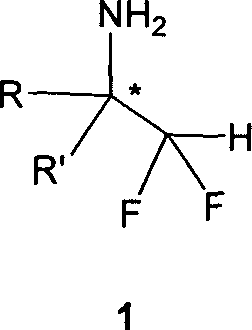
Optically pure alpha-difluoromethylamine and method of highly stereo-seective preparation
A difluoromethylamine, optical technology, applied in the field of preparation of high stereoselectivity, can solve the problems that have not been reported, and achieve the effect of short reaction time, high yield and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
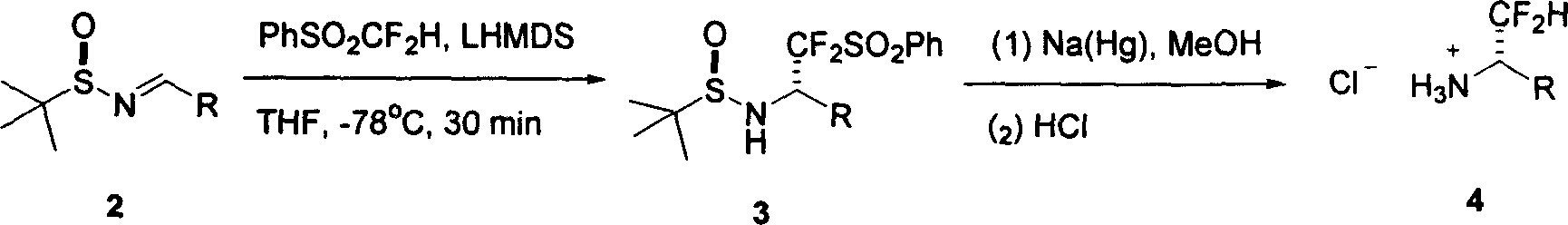
[0025] Typical preparation methods: in ether, toluene, dichloromethane, acetonitrile or tetrahydrofuran organic solvents. 2 mmol of phenyldifluoromethyl sulfone and 2 mmol of imine 2a were placed in the reaction flask. Under a dry ice-acetone bath, 2.2 mmol LHMDS was added. Stirring at this temperature for 30 min, the temperature was slowly raised to room temperature, quenched with saturated brine, and extracted with ethyl acetate (20 ml). After drying with anhydrous magnesium sulfate and spin-drying the solvent, the pure product 3a (763 mg) was obtained. Yield 95%. The characterization data of compound 3a are as follows: white solid, melting point 144-146°C; optical rotation [α] D 25 =-27.4 (c=0.8, CHCl 3). Infrared spectrum (coating method): 3066, 2962, 1584, 1449, 1349, 1158, 1087cm -1 . NMR Spectrum: 1 H NMR: δ7.89(d, J=7.5Hz, 2H), 7.70-7.75(m, 1H), 7.60(t, J=7.5Hz, 2H), 7.37-7.44(m, 5H), 5.24-5.36 (m, 1H), 4.04(d, J=7.8Hz, 1H), 1.29(s, 9H). 19 F NMR: δ-102.51 (dd...
Embodiment 2
[0036] Preparation method: 2mmol phenyl difluoromethyl sulfone and 2mmol imine 2b were placed in a reaction flask. Under a dry ice-acetone bath, 2.2 mmol LHMDS was added. Stirring at this temperature for 30 min, the temperature was slowly raised to room temperature, quenched with saturated brine, and extracted with ethyl acetate (20 ml). After drying with anhydrous magnesium sulfate and spinning to dry the solvent, the pure product 3 was obtained with a yield of 96%. The characterization data of compound 3b are as follows: white solid, melting point 108-110°C. Optical rotation [α] D 25 =-20.7 (c=0.7, CHCl 3 ). Infrared spectrum (coating method): 2961, 1612, 1585, 1516, 1158cm -1 . NMR Spectrum: 1 H NMR: δ7.88(d, J=7.5Hz, 2H), 7.68-7.73(m, 1H), 7.55(t, J=7.5Hz, 2H), 7.35(d, J=8.7Hz, 2H), 6.88(d, J=8.7Hz, 2H), 5.18-5.29(m, 1H), 3.95(d, J=8.4Hz, 1H), 3.79(s, 3H), 1.27(s, 9H). 19 FNMR: δ-102.84 (dd, J=236.3, 11.0Hz, 1F), -108.10 (dd, J=236.3, 15.8Hz, 1F). 13 C NMR: δ160.44...
Embodiment 3
[0039] The preparation method is the same as in Examples 1 and 2. The characterization data of compound 3c are as follows: white solid. Mp135-137°C. Optical rotation [α] D 25 =-17.8 (c=0.6, CHCl 3 ). Infrared spectrum (film): 2962, 1584, 1494, 1448, 1349, 1158cm -1 . NMR Spectrum: 1 H NMR: δ7.90(d, J=7.8Hz, 2H), 7.74(t, J=7.5Hz, 1H), 7.59(t, J=7.8Hz, 2H), 7.34-7.41(m, 4H), 5.22-5.34(m, 1H), 4.06(d, J=8.7Hz, 1H), 1.29(s, 9H). 19 F NMR: δ-102.49 (dd, J=238.0, 9.6Hz, 1F), -108.88 (dd, J=238.0, 18.0Hz, 1F). 13 C NMR: δ135.68, 135.39, 133.08, 131.74, 130.40, 130.07, 129.27, 129.06, 120.71 (t, J=292.5Hz), 60.12 (dd, J=24.2, 19.7Hz), 57.42, 22.29. Mass Spectrum: ( ESI, m / z): 436 (M + +1). Elemental analysis: theoretical value C 18 h 20 CIF 2 NO 3 S 2 : C, 49.59; H, 4.62; N, 3.21; Experimental values: C, 49.57; H, 4.72; N, 3.04.
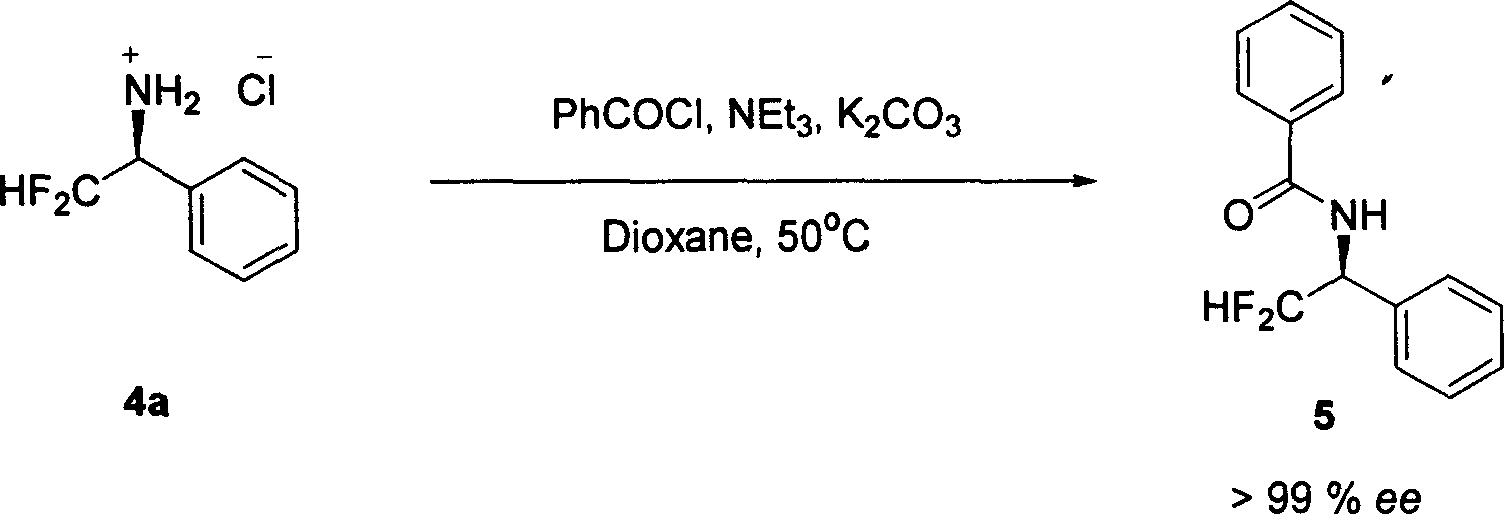
[0040] The characterization data of compound 4c are as follows: white solid. Optical rotation: [α] D 25 =24.9 (c=0.5, CH 3 OH). Infrared sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com