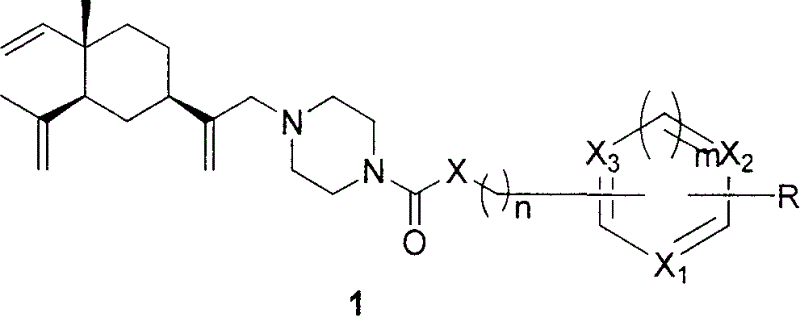
Azo hetercyle beta-elemene derivative and its preparation method and uses
A technology of elemenamide and derivatives is applied in the application field of the nitrogen-containing heterocyclic β-elemene derivatives to achieve the effects of good water solubility, high biological activity and improved biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
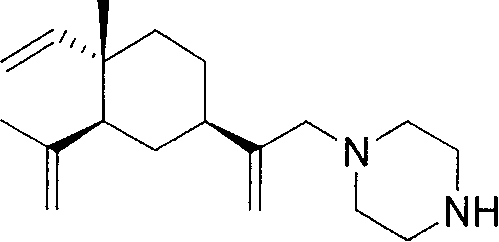
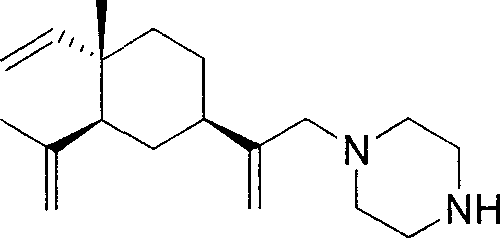
[0057] The preparation of example 1β-elemene piperazine intermediate
[0058] 1) Put 20.4g (0.1mol) of β-elemene into a 500ml jacketed three-necked bottle with a thermometer, add 9g (0.15mol) of glacial acetic acid and 80ml of dichloromethane. The reaction solution was cooled to 0-5°C, and 148 g of NaOCl aqueous solution was added dropwise. After reacting for 8h, the organic layer was separated, the aqueous layer was saturated with NaHCO3, extracted with ether (2×70ml), the combined organic layers were dried over anhydrous Na2SO4. The solvent was removed in vacuum on a water bath to obtain a light yellow oily liquid which was the monochlorinated product of β-elemene as two isomers with a content of 48.7%. Fast column separation to obtain a monochlorinated mixture of two isomers, which can be used for the next reaction without separation.
[0059] 2) Dissolve 0.86g (0.01mol) of piperazine in 20ml of dichloromethane, place in a jacketed bottle, cool to -10--5°C, protect with a...
example 2
[0062] The general synthetic method of example 2 nitrogen-containing heterocyclic β-elemenamide derivatives
[0063] The 4th) step product β-elemene piperazine intermediate and triethylamine in embodiment 1 are mixed with the dichloromethane mixed solution of 0.5mol / l, get this solution 2ml, shake at room temperature half an hour, cooling reaction system to 0-5°C. Add 2ml of 0.5mol / l acid chloride. After the reaction, 3ml of saturated NaHCO3 aqueous solution was added, shaken at room temperature for 30min, and the organic layer was separated. The organic layer was washed with water 3×3ml. Anhydrous Na2SO4 drying, vacuum precipitation, the product was obtained.
Embodiment 3
[0064] Example 3 General Synthetic Method of Nitrogen-Containing Heterocyclic β-Elemene Alkyl Derivatives
[0065] The 4th in embodiment 1) step product β-elemene piperazine intermediate and triethylamine are mixed with the toluene mixed solution of 0.5mol / l, get this solution 2ml, add the aryl chloride of 0.5mol / l or Aryl bromide 2ml. Heated to reflux for 24 hours. After the reaction, 3ml of saturated NaHCO3 aqueous solution was added, shaken at room temperature for 30min, and the organic layer was separated. The organic layer was washed with water 3×3ml. Anhydrous Na2SO4 drying, vacuum precipitation, the product was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com