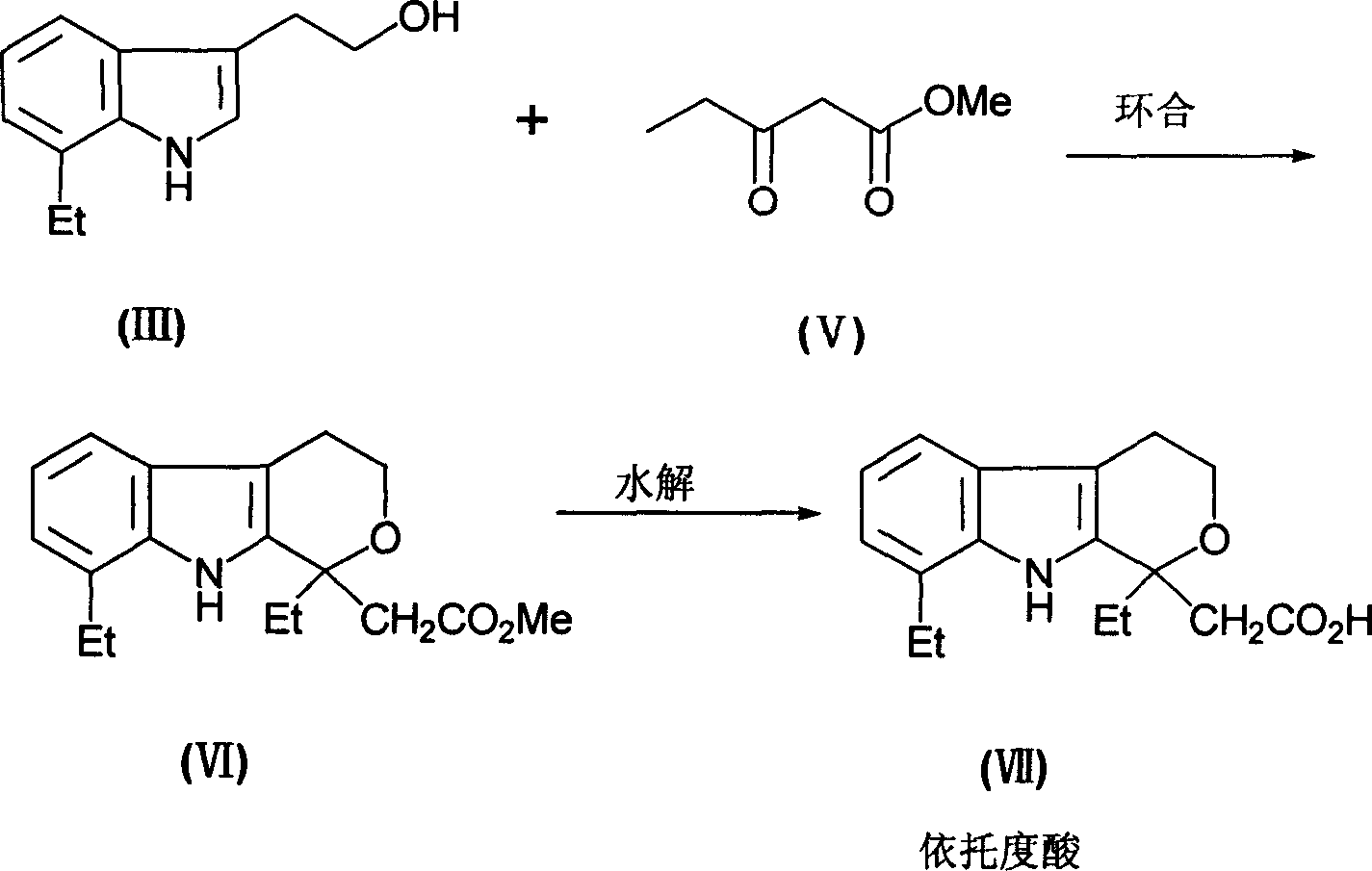
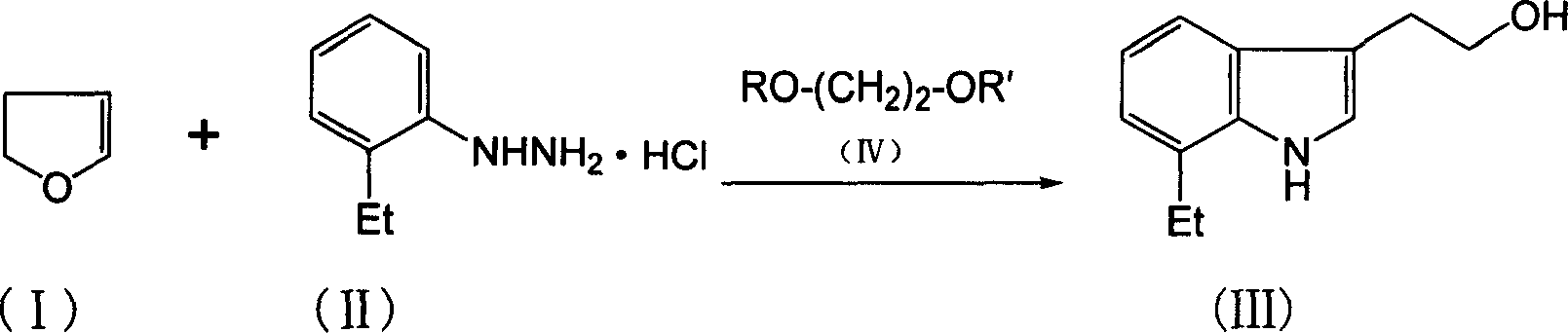Prepn process of 7-ethyl tryptophol
A technology of ethyl tryptophan and glycol ether, which is applied in the direction of organic chemistry, can solve the problems of high production cost, complicated process, and low reaction yield, and achieve the effects of convenient operation, good yield, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 7-ethyltryptol
[0019] In a 500 mL reaction flask, add 70 grams of 2,3-dihydrofuran and 350 grams of ethylene glycol monomethyl ether, stir well, and set aside.
[0020] In another 1500mL reaction flask, add 350 grams of ethylene glycol monomethyl ether, 18 grams of water, and 173 grams of o-ethylphenylhydrazine hydrochloride, heat to 80 ° C, and then, the above-mentioned 2,3-dihydro The ethylene glycol monomethyl ether solution of furan was added dropwise to the reaction solution. After the addition was completed, the reaction was carried out for 6 hours. The ethylene glycol monomethyl ether was concentrated and evaporated, and 600 grams of water and 300 grams of dichloromethane were added. After stirring and layering, the Concentrate dichloromethane, distill under reduced pressure, collect fractions: 180-200°C (5mmHg), obtain 106 grams of light yellow solid, melting point: 55-62°C, purity: 91.8% (GC), pure yield: 51.6% .
Embodiment 2~12
[0022] Change the raw material consumption and the condition of reaction shown in embodiment 1, thus the product result that obtains is as shown in table 1.
[0023]
Embodiment 13-20
[0025] Change the kind of glycol ether solvent, others are all the same as embodiment 1, and reaction result is as shown in table 2.
[0026]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com