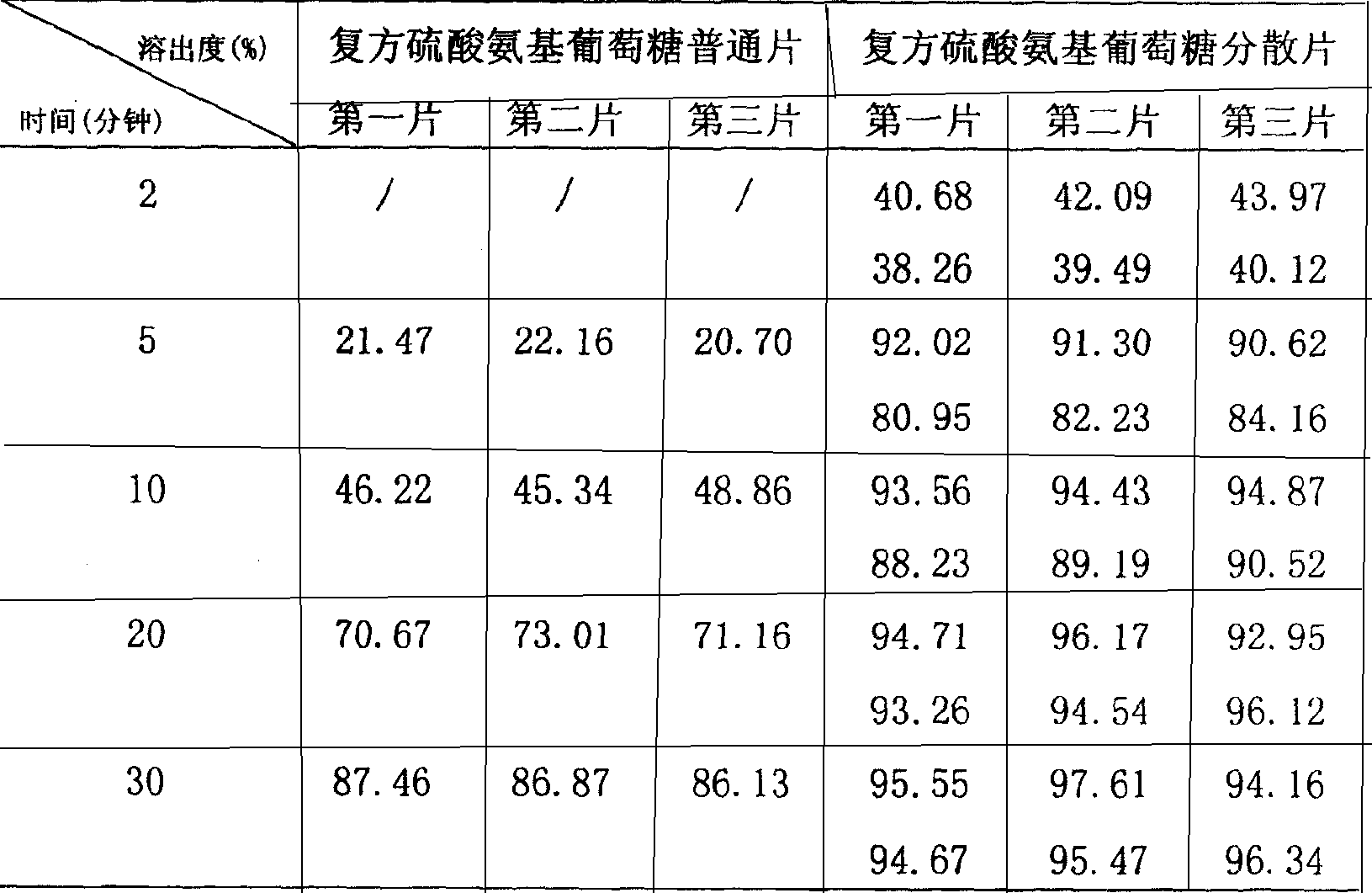
Compound glucosamine sulfate dispersible tablet formulation and its preparation method
A technology of glucosamine sulfate and dispersible tablets, which is applied in the field of medicine and can solve problems such as affecting the full effect of the drug and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The formula of dispersible tablet is;
[0048] Glucosamine Sulfate Sodium Chloride Double Salt 314g
[0049] Chondroitin Sulfate 200g
[0050] Starch 50g
[0051] 100g microcrystalline cellulose
[0052] Crospovidone (internal type) 350g
[0053] Crospovidone (external type) 350g
[0055] 16 grams of absolute ethanol
[0056] A total of 1000 tablets were made, each weighing 1.4 grams.
[0057] Glucosamine sulfate in 314 grams of glucosamine sulfate sodium chloride double salt is 250 grams.
[0058] Preparation steps: each raw material is passed through an 80-mesh sieve and pulverized; glucosamine sulfate sodium chloride double salt, chondroitin sulfate, starch, microcrystalline cellulose and crospovidone (internal addition type) are placed in a container and fully stirred and mixed Mix well, then add absolute ethanol solution for wetting, pass through 20 mesh to granulate, and dry at 45°C for 75 minutes; add the remaining crospovi...
Embodiment 2
[0060] The formula of dispersible tablet is;
[0061] Glucosamine Sulfate Sodium Chloride Double Salt 314g
[0062] Chondroitin Sulfate 196g
[0063] Starch 55g
[0064] Microcrystalline Cellulose 105g
[0065] Crospovidone (internal type) 340g
[0066] Crospovidone (external type) 350g
[0068] 18 grams of absolute ethanol
[0069] A total of 1000 tablets were made, each weighing 1.4 grams.
[0070] Preparation steps: each raw material is passed through an 80-mesh sieve and pulverized; glucosamine sulfate sodium chloride double salt, chondroitin sulfate, starch, microcrystalline cellulose and crospovidone (internal addition type) are placed in a container and fully stirred and mixed Mix well, then add absolute ethanol solution for wetting, pass through 24 mesh to granulate, and dry at 40°C for 90 minutes; add the remaining crospovidone (additional type) and magnesium stearate, and then granulate at 24 mesh; mix well , Determining the co...
Embodiment 3
[0072] The formula of dispersible tablet is;
[0073] Glucosamine Sulfate Sodium Chloride Double Salt 314g
[0074] Chondroitin Sulfate 206g
[0075] Starch 50g
[0076] Microcrystalline Cellulose 94g
[0077] Crospovidone (internal type) 345g
[0078] Crospovidone (external type) 355g
[0080] 16 grams of absolute ethanol
[0081] A total of 1000 tablets were made, each weighing 1.4 grams.
[0082] Preparation steps: each raw material is passed through an 80-mesh sieve and pulverized; glucosamine sulfate sodium chloride double salt, chondroitin sulfate, starch, microcrystalline cellulose and crospovidone (internal addition type) are placed in a container and fully stirred and mixed Mix well, then add absolute ethanol solution for wetting, pass through 24 mesh to granulate, and dry at 50°C for 60 minutes; add the remaining crospovidone (additional type) and magnesium stearate, and then granulate at 24 mesh; mix well , Determining the con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com