Dipropyl phthalic ester hapten derivant and its preparation method
A technology of dipropyl phthalate and nitrodipropyl phthalate, which is applied in the field of dipropyl phthalate hapten derivatives and its preparation, can solve the problems that do not involve dipropyl phthalate Ester hapten derivatives, whole antigens and immunoassays, etc., to achieve the effect of simple synthesis and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
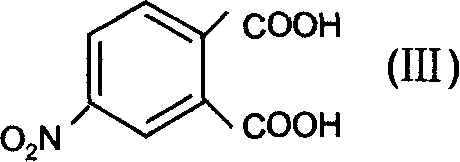
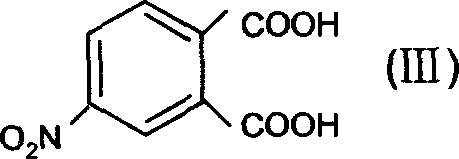
[0050] Synthesis of 4-Nitrodipropylphthalate (Step A)
[0051] Add n-propanol to 4-nitrophthalic acid, the ratio of the two substances is 1:6, add concentrated H under stirring condition 2 SO 4 Catalytic, 4-nitrophthalic acid with concentrated H 2 SO 4 The ratio of the amount of the substance is 1:0.6. After stirring and refluxing at 110° C. for 7 hours, unreacted propanol and generated water are distilled off. Pour into ice water while hot, and use 10% Na 2 CO 3 After washing until the water layer was colorless (pH = 7-8), recrystallization with absolute ethanol gave a yellow oily liquid with a yield of Y = 88%.
[0052] UV: λ 1 =214nm,λ 2 = 258nm.
[0053] IR (KBr, υ): 1529, 1357 (-NO 2 ), 1735 (C=O), 1281, 1128 (C-O-C), 2968 (-CH 3 ), 2875 (-CH 3 ), 1430 (δ-OCH 2 -), 1611 (C=C) cm -1 .
[0054] 1 H NMR (300MHz, CDCl 3 ): δ8.56(d, 1H, ArH), 8.35(dd, 1H, ArH), 7.82(d, 1H, ArH), 4.30(t, 2H, OCH 2 CH 2 CH 3 ), 4.26(t, 2H, OCH 2 CH 2 CH 3 ), 1.82-1.77 (m, 2...
Embodiment 2
[0061] Synthesis of 4-Nitrodipropylphthalate (Step A)
[0062] Add n-propanol to 4-nitrophthalic acid, the ratio of the two substances is 1:3, add dry HCl to catalyze under stirring conditions, the amount of substances between 4-nitrophthalic acid and dry HCl The ratio of the mixture was 1:0.3, and after stirring and refluxing at 100°C for 20 hours, unreacted propanol and generated water were distilled off. Pour it into cold water at 5°C while it is hot, and use 10% Na 2 CO 3 After washing until the water layer was colorless (pH = 7-8), recrystallization with absolute ethanol gave a yellow oily liquid with a yield of Y = 83%. UV: λ 1 =214nm,λ 2 = 258nm. IR (KBr, υ): 1528, 1356 (-NO 2 ), 1735 (C=O), 1281, 1128 (C-O-C), 2968 (-CH 3 ), 2875 (-CH 3 ), 1430 (δ-OCH 2 -), 1612 (C=C) cm -1 ;
[0063] Synthesis of dipropyl 4-aminophthalate (step B)
[0064] 4-Nitrodipropyl phthalate is dissolved in benzene, and pure iron powder is added, and the ratio of substances between ...
Embodiment 3
[0066] Synthesis of 4-Nitrodipropylphthalate (Step A)
[0067] Add n-propanol to 4-nitrophthalic acid, the ratio of the two substances is 1:10, add concentrated H under stirring condition 2 SO 4 Catalytic, 4-nitrophthalic acid with concentrated H 2 SO 4 The ratio of the amount of the substance is 1:0.9. After stirring and refluxing at 180° C. for 5 hours, unreacted propanol and generated water are distilled off. Pour it into cold water at 0°C while it is hot, and use 10% Na 2 CO 3 After washing until the water layer was colorless (pH = 7-8), recrystallization with absolute ethanol gave a yellow oily liquid with a yield of Y = 85%. UV: λ 1 =214nm,λ 2 = 258nm. IR (KBr, υ): 1531, 1358 (-NO 2 ), 1735 (C=O), 1280, 1129 (C-O-C), 2968 (-CH 3 ), 2875 (-CH 3 ), 1430 (δ-OCH 2 -), 1610 (C=C) cm -1 .
[0068] Synthesis of dipropyl 4-aminophthalate (step B)
[0069] 4-Nitrodipropylphthalate is dissolved in petroleum ether, and pure magnesium powder is added, and the ratio of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com