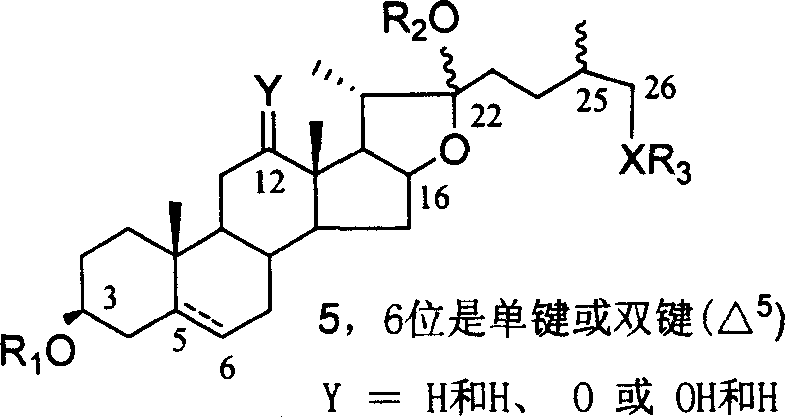
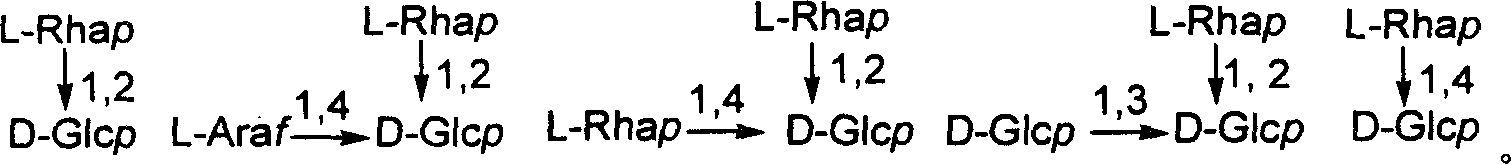
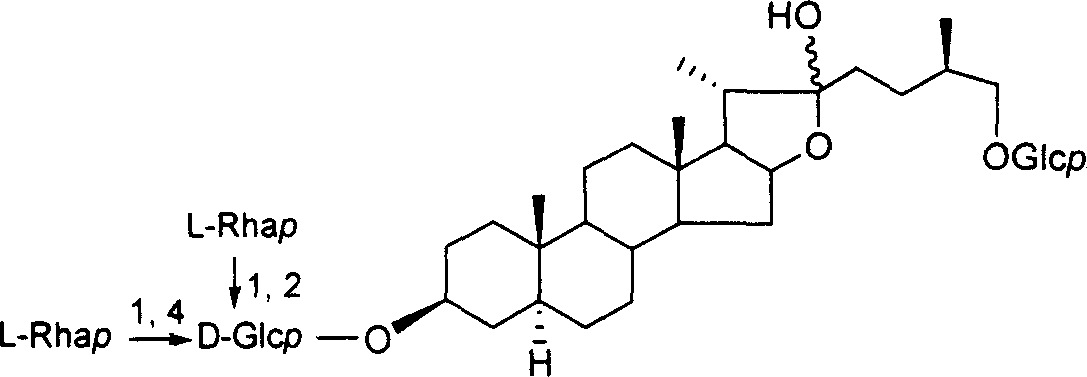
Chemical synthesis method of franosterol saponin and its derivative
A technology of furostanol saponin and chemical method, which is applied in the field of chemical synthesis of furostanol saponin and its derivatives, and can solve problems such as limiting the structure-activity relationship of compounds, restricting development and utilization, separation and purification, and partial degradation difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The following examples will help to understand the present invention, but do not limit the content of the present invention.
[0048] Synthetic example
[0049] Synthesis of 26-thiofurostanol saponin VIII:
[0050]
[0051] Reaction scheme 1: Synthesis of 26-position thiofurostanol saponin VIII
[0052] Reagents and conditions: a) 4 Å MS, trimethylsilyl triflate (TMSOTf), CH 2 Cl 2 , rt,; b) oxone, acetone, H 2 O, C 1 -C 6 Halogenated hydrocarbon, rt; c) AlI 3 , CH 3 CN, -40-80°C; d) MeONa, MeOH, CH 2 Cl 2 , -78-40°C; e) NaBH 4 , i-PrOH, CH 2 Cl 2 , rt; f) NaOH, CH 3 OH, H 2 O, 60°C
[0053] (1) Under glycosidation conditions, diosgenin reacts with 2,2,2-trimethylacetyl (Piv)-protected glucose trichloroimide ester donor I to obtain fully protected spirosteroid saponin II; (2 ) II is oxidized with oxone to obtain the product III of the double bond and 16-position oxidation; (3) III and AlI 3 The reaction obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com